
Find the product X
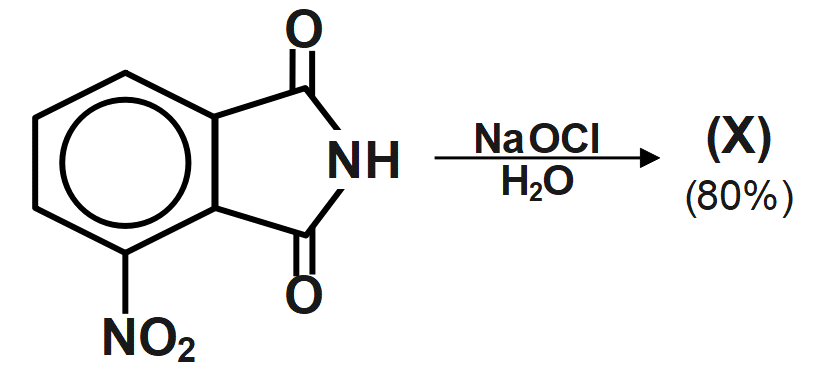
(A)
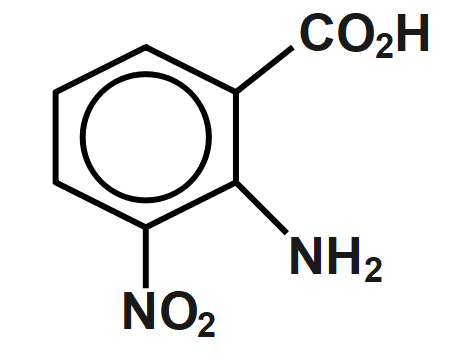
(B)
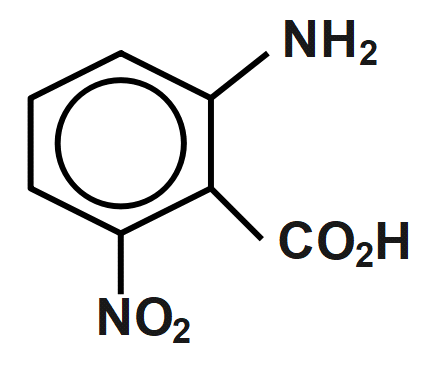
(C)
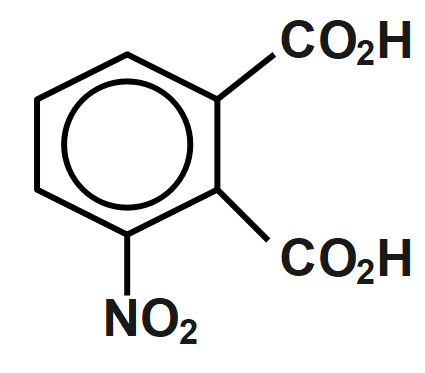
(D)
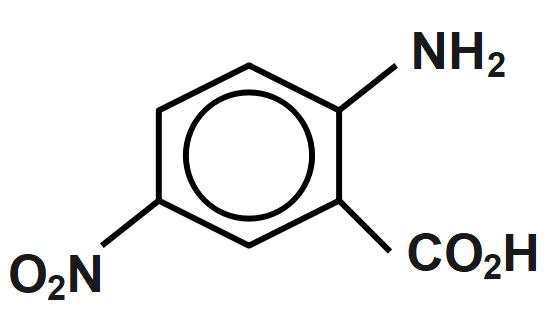





Answer
537k+ views
Hint : $ N{{H}_{3}} $ or ammonia is a colorless, foul-smelling gas. The molecular weight of the gas is $ 17 $ and it reacts with sodium hypochlorite or to form three different products.
Complete Step By Step Answer:
When ammonia reacts with sodium hypochlorite, the nitrogen atom in ammonia is oxidized to form hydrazine and the chlorine atom in sodium hypochlorite is reduced to form sodium chloride along with the formation of water molecules.
The ammonia gas acts as the reducing agent here which itself gets oxidized as its oxidation state changes from $ -3 $ to $ -2 $ while the oxidation state of chlorine in sodium hypochlorite changes from $ +1 $ to $ -1 $ in sodium chloride. The balance equation can be written as follows:
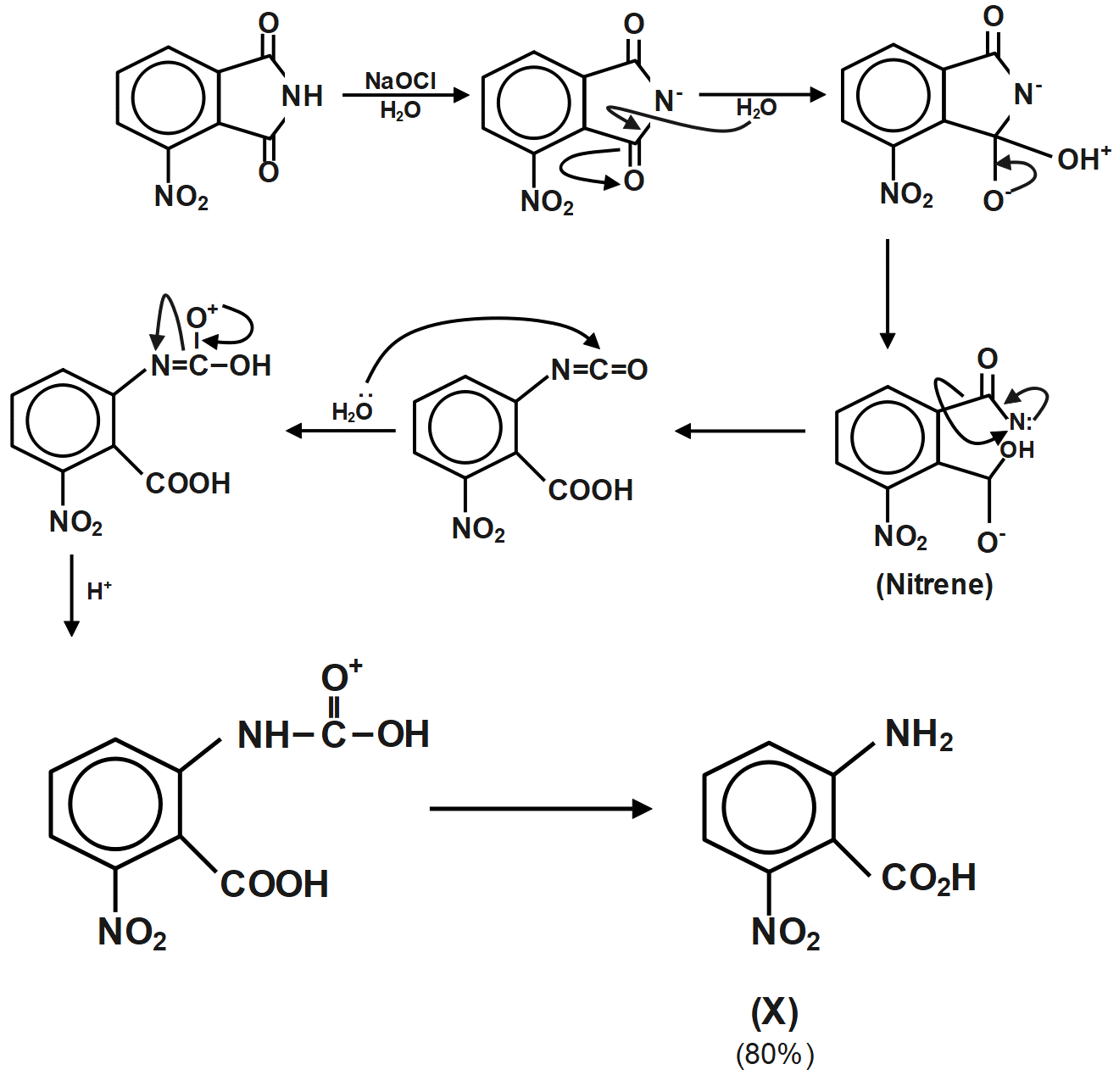
Therefore, the correct answer is option B.
Note:
There are many reactions in which ammonia acts as the reducing agent. It has this property because nitrogen is present in the and it is the lowest oxidation state for nitrogen. Hence it can lose electrons from this oxidation state to form other compounds of nitrogen. It can recover metals from metal oxides such as the reaction with cupric oxide where the copper ions in the oxide are reduced to metallic copper. It reacts with oxygen to form nitric oxide gas and this reaction is the primary step towards the formation of the nitric acid, where the nitric oxide further reacts with oxygen to form nitrogen dioxide and is then hydrated to form the nitric acid.
Complete Step By Step Answer:
When ammonia reacts with sodium hypochlorite, the nitrogen atom in ammonia is oxidized to form hydrazine and the chlorine atom in sodium hypochlorite is reduced to form sodium chloride along with the formation of water molecules.
The ammonia gas acts as the reducing agent here which itself gets oxidized as its oxidation state changes from $ -3 $ to $ -2 $ while the oxidation state of chlorine in sodium hypochlorite changes from $ +1 $ to $ -1 $ in sodium chloride. The balance equation can be written as follows:

Therefore, the correct answer is option B.
Note:
There are many reactions in which ammonia acts as the reducing agent. It has this property because nitrogen is present in the and it is the lowest oxidation state for nitrogen. Hence it can lose electrons from this oxidation state to form other compounds of nitrogen. It can recover metals from metal oxides such as the reaction with cupric oxide where the copper ions in the oxide are reduced to metallic copper. It reacts with oxygen to form nitric oxide gas and this reaction is the primary step towards the formation of the nitric acid, where the nitric oxide further reacts with oxygen to form nitrogen dioxide and is then hydrated to form the nitric acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




