
Find the sum of oxidation states in phosphorus in ${H_3}P{O_4}$ and ${H_3}P{O_3}$.
Answer
588k+ views
Hint: Oxidation state is the charge on an atom which it shows when bonded with other elements.
Complete step by step answer: We know that oxidation state of an element is known as the charge which an atom the element has in its ion or appears to have when present in the combined state with other atoms, oxidation state is also known as oxidation number. For finding oxidation number of an element, we have to follow some rules that is the oxidation number of all the atoms in their elemental state (for example :- ${N_2},C{l_2},{H_2},He,{P_4},{S_8},{O_2}$) is zero. The oxidation number of monatomic ions is the same as the charge on it. For example:-Oxidation number of $N{a^ + },M{g^{2 + }}$ are +1 and +2 respectively. The oxidation number of hydrogen is +1 when combined with non-metal and -1 when combined with active metals. The oxidation number of oxidation is -2 in most of its compounds, except in peroxide.
Now we have the general overview of oxidation state. Now let’s calculate the oxidation state of phosphorus in ${H_3}P{O_4}$. The ${H_3}P{O_4}$ has the following structure:-
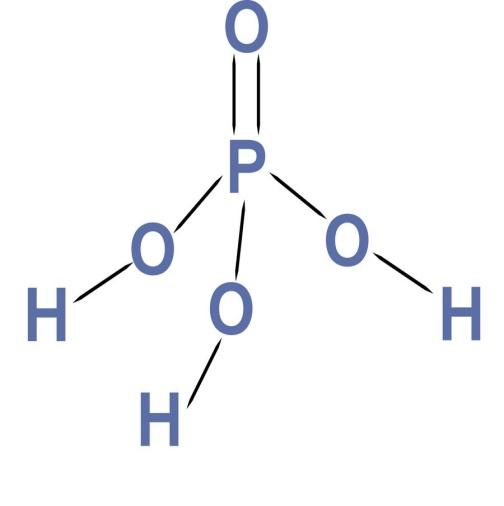
Let the oxidation state of phosphorous be x. As we know that the oxidation state of hydrogen is +1. Since we have hydrogen atom so the combined state of these 3 hydrogen is 3. The oxidation state of oxygen is -2. Since we have 4 oxygen atoms, the combined oxidation state of oxygen is -8. Since the compound is whole electrically neutral, so the combined oxidation state of the whole atom is zero.
X+3-8=0
Or x=-5
Hence the oxidation state of phosphorus in ${H_3}P{O_4}$ is -5.
Now let’s calculate the oxidation state of phosphorus in ${H_3}P{O_3}$. The ${H_3}P{O_3}$ has the following structure.
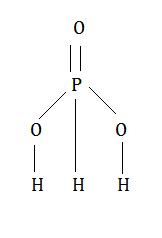
Now consider the oxidation state of phosphorus be y. Since we have 3 hydrogen and oxygen atoms , combined oxidation states of is -6. Since the compound as a whole is neutral so oxidation states of all elements must be zero.
Y+3-6=0
That is y=3
Therefore the sum of oxidation states of phosphorus in ${H_3}P{O_4}$ and ${H_3}P{O_3}$ is x+y that is 5+3=8.
Hence sum of oxidation state of phosphorus in ${H_3}P{O_4}$ and ${H_3}P{O_3}$ is $8.$
Note: Oxygen commonly shows the oxidation state -2 except in its peroxide compounds where it’s oxidation state is -1. That’s why we have to firstly draw the compound structure and then solve these types of questions.
Complete step by step answer: We know that oxidation state of an element is known as the charge which an atom the element has in its ion or appears to have when present in the combined state with other atoms, oxidation state is also known as oxidation number. For finding oxidation number of an element, we have to follow some rules that is the oxidation number of all the atoms in their elemental state (for example :- ${N_2},C{l_2},{H_2},He,{P_4},{S_8},{O_2}$) is zero. The oxidation number of monatomic ions is the same as the charge on it. For example:-Oxidation number of $N{a^ + },M{g^{2 + }}$ are +1 and +2 respectively. The oxidation number of hydrogen is +1 when combined with non-metal and -1 when combined with active metals. The oxidation number of oxidation is -2 in most of its compounds, except in peroxide.
Now we have the general overview of oxidation state. Now let’s calculate the oxidation state of phosphorus in ${H_3}P{O_4}$. The ${H_3}P{O_4}$ has the following structure:-

Let the oxidation state of phosphorous be x. As we know that the oxidation state of hydrogen is +1. Since we have hydrogen atom so the combined state of these 3 hydrogen is 3. The oxidation state of oxygen is -2. Since we have 4 oxygen atoms, the combined oxidation state of oxygen is -8. Since the compound is whole electrically neutral, so the combined oxidation state of the whole atom is zero.
X+3-8=0
Or x=-5
Hence the oxidation state of phosphorus in ${H_3}P{O_4}$ is -5.
Now let’s calculate the oxidation state of phosphorus in ${H_3}P{O_3}$. The ${H_3}P{O_3}$ has the following structure.

Now consider the oxidation state of phosphorus be y. Since we have 3 hydrogen and oxygen atoms , combined oxidation states of is -6. Since the compound as a whole is neutral so oxidation states of all elements must be zero.
Y+3-6=0
That is y=3
Therefore the sum of oxidation states of phosphorus in ${H_3}P{O_4}$ and ${H_3}P{O_3}$ is x+y that is 5+3=8.
Hence sum of oxidation state of phosphorus in ${H_3}P{O_4}$ and ${H_3}P{O_3}$ is $8.$
Note: Oxygen commonly shows the oxidation state -2 except in its peroxide compounds where it’s oxidation state is -1. That’s why we have to firstly draw the compound structure and then solve these types of questions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




