
How many of the following compounds have peroxide linkage?
\[{{H}_{3}}P{{O}_{5}},\text{ }{{H}_{2}}S{{O}_{5}},\text{ }{{H}_{2}}{{S}_{2}}{{O}_{8}},\text{ }HN{{O}_{4}},\text{ }{{H}_{4}}{{P}_{2}}{{O}_{7}},\text{ }{{H}_{2}}{{S}_{2}}{{O}_{3}},\text{ }{{H}_{2}}{{S}_{2}}{{O}_{6}}\]
A. 4
B. 5
C. 6
D. None of these
Answer
569.7k+ views
Hint: If there is a chemical bond between two oxygen atoms in a molecule then the chemical bond is called peroxide bond. The peroxide bonds are very less stable in the presence of sunlight and we have to keep them away from the sunlight.
Complete answer:
- In the question it is given how many of the given compounds contain peroxide bonds.
- We have to find the number of compounds that have peroxide bonds in their structures.
- The structure of ${{H}_{3}}P{{O}_{5}}$ is as follows.
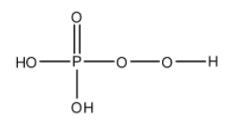
- ${{H}_{3}}P{{O}_{5}}$ has one peroxide bond in its structure.
- The structure of ${{H}_{2}}S{{O}_{5}}$ is as follows.
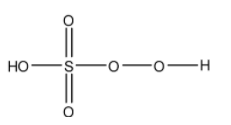
- ${{H}_{2}}S{{O}_{5}}$ has one peroxide bond in its structure.
- The structure of ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is as follows.
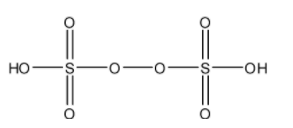
- ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ has one peroxide bond in its structure.
- The structure of $HN{{O}_{4}}$ is as follows.
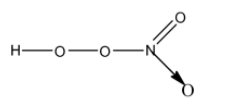
- $HN{{O}_{4}}$ has one peroxide bond in its structure.
- The structure of ${{H}_{4}}{{P}_{2}}{{O}_{7}}$ is as follows.
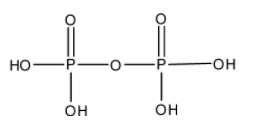
- There is no peroxide bond in ${{H}_{4}}{{P}_{2}}{{O}_{7}}$ .
- The structure of ${{H}_{2}}{{S}_{2}}{{O}_{3}}$ is as follows.
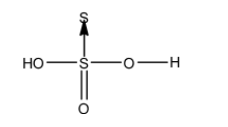
- There is no peroxide bond in ${{H}_{2}}{{S}_{2}}{{O}_{3}}$
- The structure of ${{H}_{2}}{{S}_{2}}{{O}_{6}}$ is as follows.
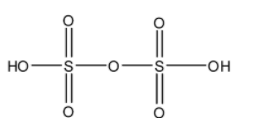
- There is no peroxide bond in ${{H}_{2}}{{S}_{2}}{{O}_{6}}$
- Out of the given seven compounds only four compounds have peroxide bonds in their structure.
So, the correct option is A.
Note:
Without drawing or knowing the structure of the compounds we cannot say whether the compound contains a peroxide bond or not. The structure of the molecules or compounds reveals the presence of the peroxide bond.
Complete answer:
- In the question it is given how many of the given compounds contain peroxide bonds.
- We have to find the number of compounds that have peroxide bonds in their structures.
- The structure of ${{H}_{3}}P{{O}_{5}}$ is as follows.

- ${{H}_{3}}P{{O}_{5}}$ has one peroxide bond in its structure.
- The structure of ${{H}_{2}}S{{O}_{5}}$ is as follows.

- ${{H}_{2}}S{{O}_{5}}$ has one peroxide bond in its structure.
- The structure of ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is as follows.

- ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ has one peroxide bond in its structure.
- The structure of $HN{{O}_{4}}$ is as follows.

- $HN{{O}_{4}}$ has one peroxide bond in its structure.
- The structure of ${{H}_{4}}{{P}_{2}}{{O}_{7}}$ is as follows.

- There is no peroxide bond in ${{H}_{4}}{{P}_{2}}{{O}_{7}}$ .
- The structure of ${{H}_{2}}{{S}_{2}}{{O}_{3}}$ is as follows.

- There is no peroxide bond in ${{H}_{2}}{{S}_{2}}{{O}_{3}}$
- The structure of ${{H}_{2}}{{S}_{2}}{{O}_{6}}$ is as follows.

- There is no peroxide bond in ${{H}_{2}}{{S}_{2}}{{O}_{6}}$
- Out of the given seven compounds only four compounds have peroxide bonds in their structure.
So, the correct option is A.
Note:
Without drawing or knowing the structure of the compounds we cannot say whether the compound contains a peroxide bond or not. The structure of the molecules or compounds reveals the presence of the peroxide bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




