
How can the following conversion be carried out?
Ethanol to propanenitrile
Answer
565.2k+ views
Hint: The product of the given conversion contains a cyanide group which is easily introduced by substitution reaction to any alkyl halide.
Complete step by step answer:
-Ethanol is an organic compound having an alcohol functional group. Ethanol is the higher homologue of methanol which is the simplest alcohol. The ethanol contains an ethyl group attached to a hydroxyl group. Thus the chemical formula of ethanol is \[C{H_3}C{H_2}OH\].
-The propanenitrile is an organic compound having a cyanide functional group. The number of carbon atoms is three in this compound. The cyanide group is a good nucleophile and can easily displace a good leaving group from a compound. Thus alkyl halides are used as starting materials to introduce cyanide group by displacement of the halide group.
-Specifically alkyl bromides or iodides are used as starting material because aliphatic bromide or iodide are better for leaving the group due to greater stabilization of the anion. The fluorides or chlorides are not good leaving groups for aliphatic halides.
-Thus the retrosynthesis for the compound is written as:
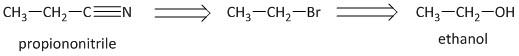
-The bromides are easily prepared from the corresponding alcohol using phosphorus tribromide. Thus the conversion from ethanol to propanenitrile is carried out in the following manner.
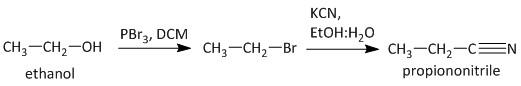
Note:
The haloalkane (\[R - X\]) is a vital intermediate in organic synthesis. Several nucleophiles can be introduced using the corresponding bromide or iodide. The Williamson ether synthesis is a very important reaction in organic chemistry for the preparation of ethers. For this the alcohols are treated with alkyl halides. Amines are also introduced in a similar way by replacing the halide group.
Complete step by step answer:
-Ethanol is an organic compound having an alcohol functional group. Ethanol is the higher homologue of methanol which is the simplest alcohol. The ethanol contains an ethyl group attached to a hydroxyl group. Thus the chemical formula of ethanol is \[C{H_3}C{H_2}OH\].
-The propanenitrile is an organic compound having a cyanide functional group. The number of carbon atoms is three in this compound. The cyanide group is a good nucleophile and can easily displace a good leaving group from a compound. Thus alkyl halides are used as starting materials to introduce cyanide group by displacement of the halide group.
-Specifically alkyl bromides or iodides are used as starting material because aliphatic bromide or iodide are better for leaving the group due to greater stabilization of the anion. The fluorides or chlorides are not good leaving groups for aliphatic halides.
-Thus the retrosynthesis for the compound is written as:
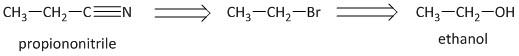
-The bromides are easily prepared from the corresponding alcohol using phosphorus tribromide. Thus the conversion from ethanol to propanenitrile is carried out in the following manner.
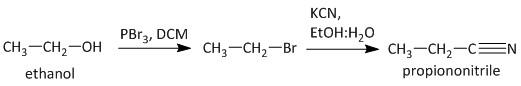
Note:
The haloalkane (\[R - X\]) is a vital intermediate in organic synthesis. Several nucleophiles can be introduced using the corresponding bromide or iodide. The Williamson ether synthesis is a very important reaction in organic chemistry for the preparation of ethers. For this the alcohols are treated with alkyl halides. Amines are also introduced in a similar way by replacing the halide group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




