
For a given principal level n=4, the energy of its sub shells is of the order:
A.$s < d < f < p$
B.$s < p < d < f$
C.$d < f < p < s$
D.$s < p < f < d$
Answer
569.1k+ views
Hint:
We need to know that the subshells are parts of shells. The energy of shells and subshells are classified by the theory of electron configuration. The electron configuration is defined as the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
Complete step by step answer:
As we know that the various elements have their unique electronic configuration. Since, the atomic number is different for all the elements likewise their electron configuration is also different.
In atoms there are two parts, one is the center (core) and the orbitals. We know that the center part contains protons and neutrons lumped together and the orbitals contain the electrons revolving around. The orbitals are classified as s, p, d and f orbitals. The filling takes place starting from s orbitals. Every shell contains subshells.
The filling of the subshells are classified by Aufbau principle. The orbitals with lowest atomic energy levels are occupied first and the rising higher atomic orbitals are filled in a built-up manner.
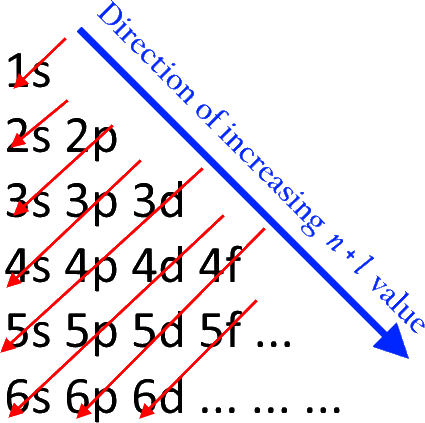
If we look at the energy level 4, we can see that the filling takes place starting from 4s, then 4p, then 4d and lastly in 4f.
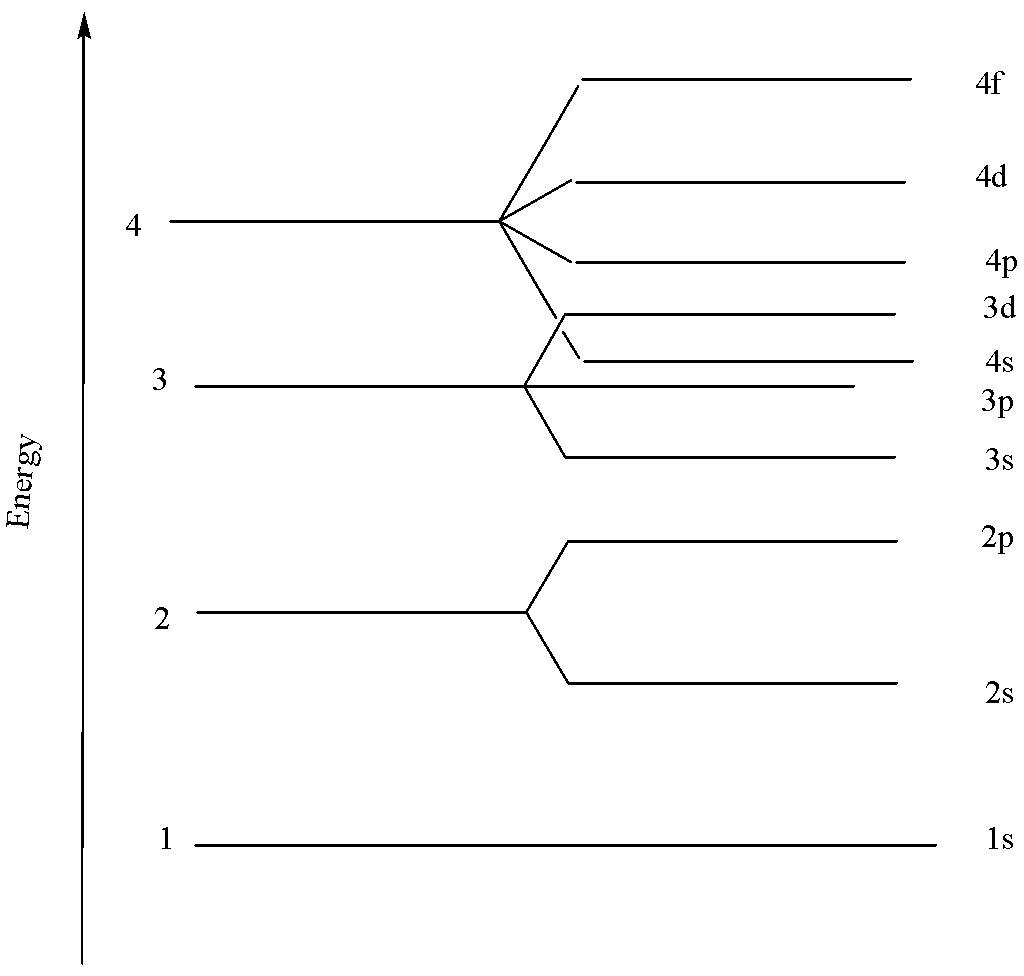
Hence, the correct answer to the question is option B. $s < p < d < f$.
Note: The study of atoms and molecules in terms of their electrons and electronic configuration is also known as study of quantum mechanics. Quantum mechanics helps to understand the nature of electrons. It is used to study the energy levels of atoms. The photon emission is a concept when a photon is emitted when an electron leaves one orbital and goes to another orbital. The stability and energy of electrons is necessary for the study of quantum mechanics.
We need to know that the subshells are parts of shells. The energy of shells and subshells are classified by the theory of electron configuration. The electron configuration is defined as the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
Complete step by step answer:
As we know that the various elements have their unique electronic configuration. Since, the atomic number is different for all the elements likewise their electron configuration is also different.
In atoms there are two parts, one is the center (core) and the orbitals. We know that the center part contains protons and neutrons lumped together and the orbitals contain the electrons revolving around. The orbitals are classified as s, p, d and f orbitals. The filling takes place starting from s orbitals. Every shell contains subshells.
The filling of the subshells are classified by Aufbau principle. The orbitals with lowest atomic energy levels are occupied first and the rising higher atomic orbitals are filled in a built-up manner.

If we look at the energy level 4, we can see that the filling takes place starting from 4s, then 4p, then 4d and lastly in 4f.

Hence, the correct answer to the question is option B. $s < p < d < f$.
Note: The study of atoms and molecules in terms of their electrons and electronic configuration is also known as study of quantum mechanics. Quantum mechanics helps to understand the nature of electrons. It is used to study the energy levels of atoms. The photon emission is a concept when a photon is emitted when an electron leaves one orbital and goes to another orbital. The stability and energy of electrons is necessary for the study of quantum mechanics.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




