
For \[Acetophenone\xrightarrow{{HC{O_3}H}}A\xrightarrow{{{H_3}{O^ + }}}B + C\xrightarrow{{Phthalic Anhydride,{H^ + }}}Indicator(D)\]
\[C\] and \[D\] are
A)
B)
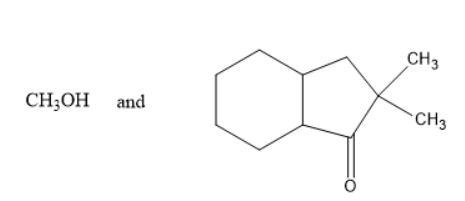
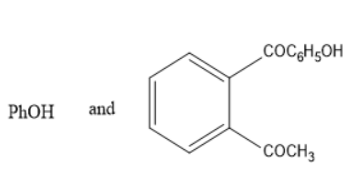
C)
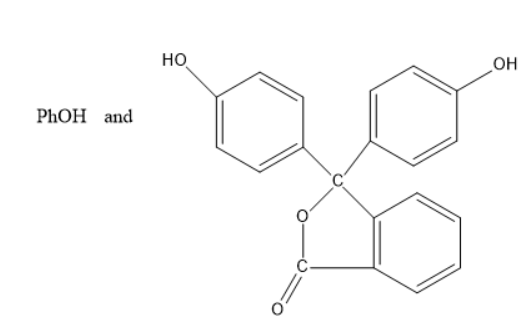
D)
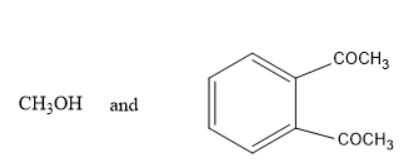




Answer
496.8k+ views
Hint: Acetophenone is a ketone, undergoes several reactions with different reagents.
Performic acid (PFA) is used for epoxidation to form an ester from acetophenone, and on acid hydrolysis forms an acid and alcohol.
This alcohol when treated with Phthalic anhydride in presence of acid catalyst forms an indicator.
Complete answer: Carbonyl compounds are the compounds containing carbonyl groups.
Aldehydes and ketones belong to carbonyl compounds.
Acetophenone is a ketone with the molecular formula of \[{C_6}{H_5}COC{H_3}\] .
Performic acid is a compound with the molecular formula \[HC{O_3}H\].
It can be used for epoxidation.
When acetophenone treated with Performic acid forms phenyl ethanoate, it an ester molecule
When phenyl ethanoate is treated with hydronium ion, it forms an acid and alcohol.
It is a reverse of the esterification process, i.e.., ester hydrolysis.
Ester hydrolysis is also known as saponification.
In ester hydrolysis, ester and hydronium ions react to give a carboxylic acid and alcohol.
The alkoxy group turned to alcohol and the carboxyl part turned into carboxylic acid.
Thus, it leads to the formation of phenol and acetic acid.
When phenol is treated with phthalic anhydride in presence of acid catalyst or acidic medium, phenolphthalein indicator is formed.
The below mechanism shows the formation of phenolphthalein indicators from acetophenone.
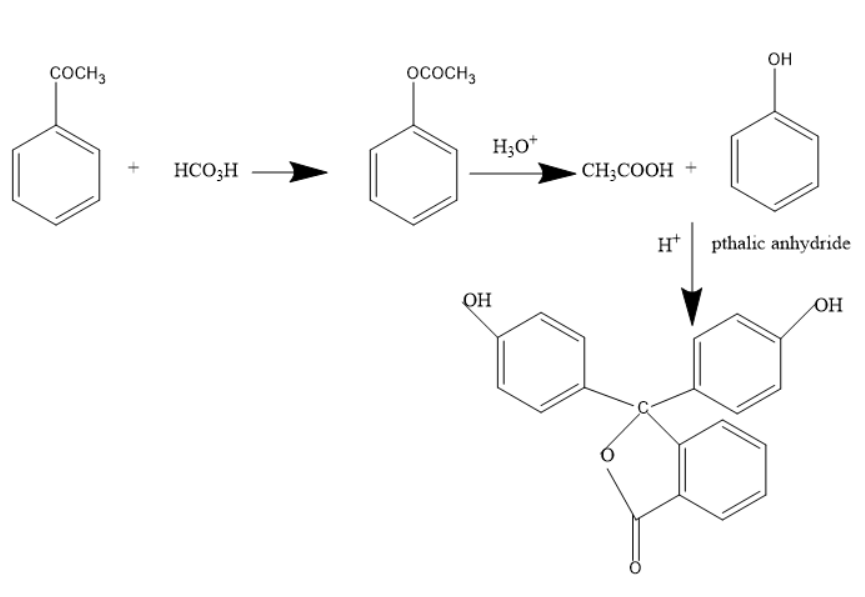
Thus, the products C and D are phenol and phenolphthalein indicators.
And hence Option C is the correct one.
In option A and D, methanol is formed which is not true as the alkoxy group must be turned into alcohol i.e.., phenol in the above reaction. Thus, it is not the product.
Note:
The phenolphthalein indicator structure must be known clearly.
The ester hydrolysis products must be clearly known and the alkoxy part turned into alcohol and the carboxyl part must be turned into carboxylic acid.
Performic acid (PFA) is used for epoxidation to form an ester from acetophenone, and on acid hydrolysis forms an acid and alcohol.
This alcohol when treated with Phthalic anhydride in presence of acid catalyst forms an indicator.
Complete answer: Carbonyl compounds are the compounds containing carbonyl groups.
Aldehydes and ketones belong to carbonyl compounds.
Acetophenone is a ketone with the molecular formula of \[{C_6}{H_5}COC{H_3}\] .
Performic acid is a compound with the molecular formula \[HC{O_3}H\].
It can be used for epoxidation.
When acetophenone treated with Performic acid forms phenyl ethanoate, it an ester molecule
When phenyl ethanoate is treated with hydronium ion, it forms an acid and alcohol.
It is a reverse of the esterification process, i.e.., ester hydrolysis.
Ester hydrolysis is also known as saponification.
In ester hydrolysis, ester and hydronium ions react to give a carboxylic acid and alcohol.
The alkoxy group turned to alcohol and the carboxyl part turned into carboxylic acid.
Thus, it leads to the formation of phenol and acetic acid.
When phenol is treated with phthalic anhydride in presence of acid catalyst or acidic medium, phenolphthalein indicator is formed.
The below mechanism shows the formation of phenolphthalein indicators from acetophenone.

Thus, the products C and D are phenol and phenolphthalein indicators.
And hence Option C is the correct one.
In option A and D, methanol is formed which is not true as the alkoxy group must be turned into alcohol i.e.., phenol in the above reaction. Thus, it is not the product.
Note:
The phenolphthalein indicator structure must be known clearly.
The ester hydrolysis products must be clearly known and the alkoxy part turned into alcohol and the carboxyl part must be turned into carboxylic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




