
For ${C_7}{H_{16}}$ has $9$ isomers how many of these isomers have quaternary carbons?
A.1
B.2
C.3
D.4
Answer
569.1k+ views
Hint:
We have to discuss here about Isomers (Isomers are molecules with the same molecular formulas, but different arrangements of atoms) and identify the number of isomers that have quaternary carbon.
Complete step by step answer:
ISOMERS: The molecular geometries of hydrocarbons are directly related to the physical and chemical properties of these molecules. Molecules that have the same molecular formula but different molecular structures are called isomers.
There are two major classes of isomers:
-Structural Isomer: In this, the atoms in each isomer are connected, or bonded, in different ways. As a result, structural isomers often contain different functional groups or patterns of bonding.
-Stereoisomers: In this, the atoms in each isomer are connected in the same way but differ in how they are oriented in space. There are two types of Stereoisomers:
A.Enantiomers are stereoisomers which are non-superimposable mirror images of each other. One of them rotates the plane polarised light towards and the other towards left.
B.Diastereomers are any stereoisomers that are not enantiomers. One common example of a diastereomer is a cis-trans isomer. It can occur when atoms or functional groups are situated on either end of a rigid carbon-carbon bond, such as a double bond.
According to the Question,
Heptane chemical formula is ${C_7}{H_{16}}$. Isomers which contain quaternary carbons are:
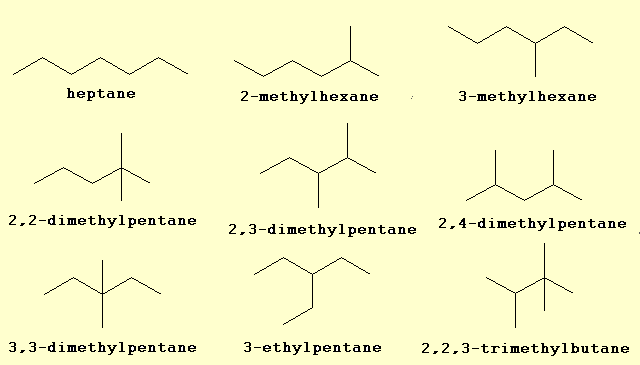
$\left( 1 \right)\,2,2 - \operatorname{di} - methylpentan e$
$\left( 2 \right)\,3,3 - di - methylpentane$
$\left( 3 \right)\,2,2,3 - tri - methylbutane$
Note: A quaternary carbon is a carbon atom bound to four other carbon atoms. For this reason, quaternary carbon atoms are found only in hydrocarbons which have at least five carbon atoms. Quaternary carbon atoms can occur in branched alkanes, but not in linear alkanes.
We have to discuss here about Isomers (Isomers are molecules with the same molecular formulas, but different arrangements of atoms) and identify the number of isomers that have quaternary carbon.
Complete step by step answer:
ISOMERS: The molecular geometries of hydrocarbons are directly related to the physical and chemical properties of these molecules. Molecules that have the same molecular formula but different molecular structures are called isomers.
There are two major classes of isomers:
-Structural Isomer: In this, the atoms in each isomer are connected, or bonded, in different ways. As a result, structural isomers often contain different functional groups or patterns of bonding.
-Stereoisomers: In this, the atoms in each isomer are connected in the same way but differ in how they are oriented in space. There are two types of Stereoisomers:
A.Enantiomers are stereoisomers which are non-superimposable mirror images of each other. One of them rotates the plane polarised light towards and the other towards left.
B.Diastereomers are any stereoisomers that are not enantiomers. One common example of a diastereomer is a cis-trans isomer. It can occur when atoms or functional groups are situated on either end of a rigid carbon-carbon bond, such as a double bond.
According to the Question,
Heptane chemical formula is ${C_7}{H_{16}}$. Isomers which contain quaternary carbons are:

$\left( 1 \right)\,2,2 - \operatorname{di} - methylpentan e$
$\left( 2 \right)\,3,3 - di - methylpentane$
$\left( 3 \right)\,2,2,3 - tri - methylbutane$
Note: A quaternary carbon is a carbon atom bound to four other carbon atoms. For this reason, quaternary carbon atoms are found only in hydrocarbons which have at least five carbon atoms. Quaternary carbon atoms can occur in branched alkanes, but not in linear alkanes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




