
For carbanion stability order will be:
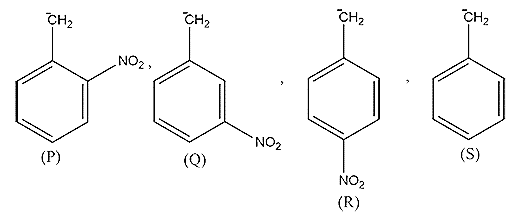
A. P ˃ Q ˃ S ˃ R
B. P ˃ R ˃ Q ˃ S
C. Q ˃ R ˃ S ˃ P
D. S ˃ R ˃ Q ˃ P

Answer
588k+ views
Hint: Carbanion is the carbon atom which has an extra pair of the electron due to which it consists of negative charge. The stability of carbanion depends on two factors mainly i.e. the s - character and the position of electron-withdrawing or electron-donating group.
Complete step by step answer:
-There are 3 positions at which the group other than functional group attaches i.e. ortho, para and meta position.
-Ortho position is the position which comes after the functional group.
-Meta position is adjacent to the ortho position whereas the para position is adjacent to the meta.
-For example, in structure P nitro group is attached to the ortho position whereas, in Q, the nitro group is attached to the meta group and in R, the nitro group is attached to the para position.
-Now, we know that a nitro group is an electron-withdrawing group (Those species which tends to accept a pair of an electron and decreases the electron density) , so it will stabilize the carbanion.
-So, from the given structure S will have the least stable carbanion because it doesn't have a nitro or electron-withdrawing group.
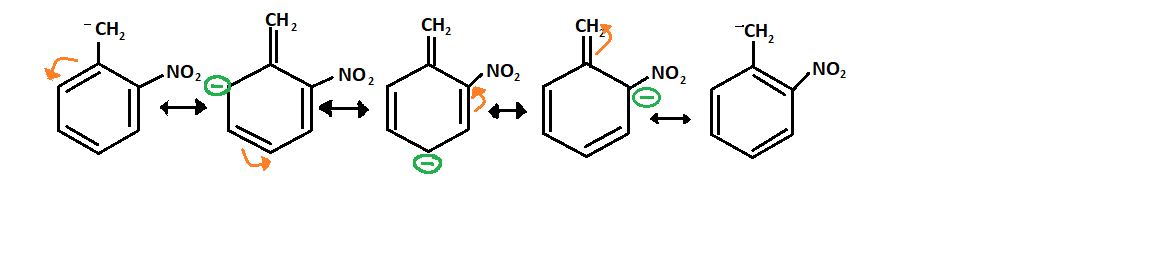
-Now, in structure P, the nitro group is at ortho position so it will be the most stable because here resonance is more effective and makes the carbanion stable.
Resonance of structure P:
Resonance structure of R:
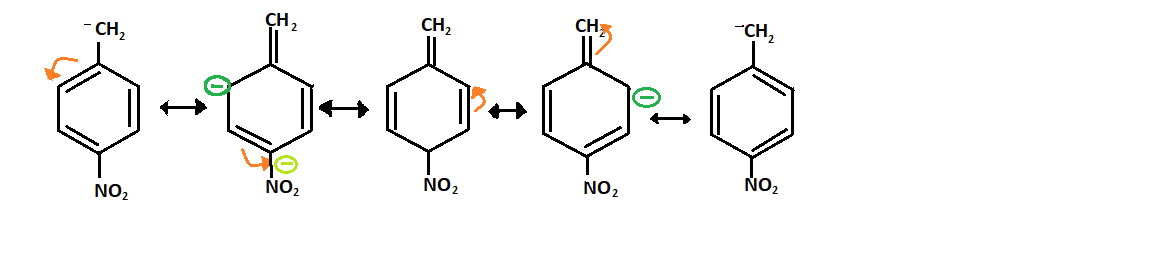
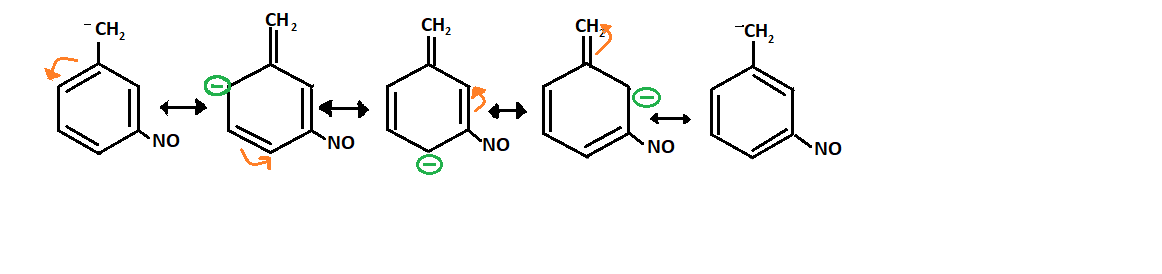
-As it is present adjacent to the carbanion so it will have more stability than para-nitro because it will decrease more negative ion on the carbanion and forms the resonance structure:
-Whereas the stability of R will be less than P and Q because the nitro group is present at meta position due to which there is less resonance effect as given below:
Resonance structure of Q:
So, the correct answer is “Option B”.
Note: Electron donating groups such as hydroxyl group, alkyl group makes the carbanion unstable because they tend to donate the pair of an electron to the carbon atom and increases the electron density on the carbanion making it more unstable.
Complete step by step answer:
-There are 3 positions at which the group other than functional group attaches i.e. ortho, para and meta position.
-Ortho position is the position which comes after the functional group.
-Meta position is adjacent to the ortho position whereas the para position is adjacent to the meta.
-For example, in structure P nitro group is attached to the ortho position whereas, in Q, the nitro group is attached to the meta group and in R, the nitro group is attached to the para position.
-Now, we know that a nitro group is an electron-withdrawing group (Those species which tends to accept a pair of an electron and decreases the electron density) , so it will stabilize the carbanion.
-So, from the given structure S will have the least stable carbanion because it doesn't have a nitro or electron-withdrawing group.
-Now, in structure P, the nitro group is at ortho position so it will be the most stable because here resonance is more effective and makes the carbanion stable.
Resonance of structure P:

Resonance structure of R:

-As it is present adjacent to the carbanion so it will have more stability than para-nitro because it will decrease more negative ion on the carbanion and forms the resonance structure:
-Whereas the stability of R will be less than P and Q because the nitro group is present at meta position due to which there is less resonance effect as given below:
Resonance structure of Q:

So, the correct answer is “Option B”.
Note: Electron donating groups such as hydroxyl group, alkyl group makes the carbanion unstable because they tend to donate the pair of an electron to the carbon atom and increases the electron density on the carbanion making it more unstable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




