
For ‘invert sugar’, the correct statement(s) is (are)-
(Given: Specific rotations of (+)-sucrose, (+)-maltose, L-(-)-glucose and L-(+)-fructose in aqueous solution are \[ + {66^\circ }\], $ + {140^\circ }$,$ - {52^\circ }$ and ${92^\circ }$, respectively)
A. ‘Invert sugar’ is prepared by acid catalyzed hydrolysis of maltose.
B. ‘Invert sugar’ is an equimolar mixture of D-(+)-glucose and D-(-)-fructose.
C. Specific rotation of invert sugar is $ - {20^\circ }$.
D. In reaction with $B{r_2}$ water, ‘invert sugar’ forms saccharic acid as one of the products.
Answer
569.4k+ views
Hint: Sucrose is white crystalline solid. It is the most common sugar used in food. It is a sweetening agent which is used in jams, in cold drinks. It is a disaccharide made up of two monosaccharide glucose and fructose. It is dextrorotatory but hydrolysis gives an equimolar mixture of dextro and levo form of its constituents.
Complete step by step answer:
The chemical formula of sucrose is ${C_{12}}{H_{22}}{O_{11}}$. Its structure is given as-
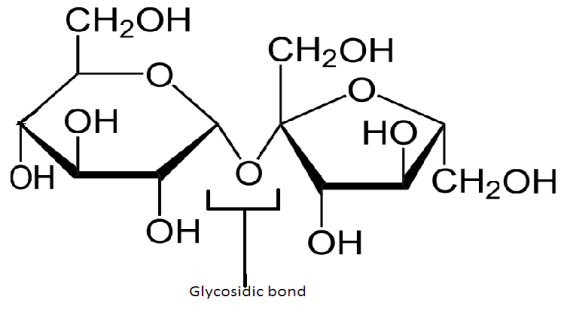
It occurs in nature Sucrose is called invert sugar because on hydrolysis with acid or enzyme, it gives equal parts of glucose and fructose which constitute the two monosaccharide units of sucrose. The reaction is given as-
${C_{12}}{H_{22}}{O_{11}} + {H_2}O \to \mathop {{C_6}{H_{12}}{O_6}}\limits_{D( + ) - {\text{glucose}}} + \mathop {{C_6}{H_{12}}{O_6}}\limits_{D( - ) - {\text{fructose}}} $
Sucrose is dextrorotatory with specific rotation \[ + {66^\circ }\] but it changes to laevorotatory due to formation of one mole of glucose and one mole of fructose which have specific rotations $ + {52^\circ }$ and $ - {92^\circ }$ respectively.
Net specific rotation is the average of specific rotation of glucose and fructose molecule as both monomers are present in equal amount so we can write-
$ \Rightarrow $ $\alpha = \dfrac{{52 + \left( { - 92} \right)}}{2}$
On solving, we get-
$ \Rightarrow $ $\alpha = - \dfrac{{40}}{2} = - {20^\circ }$
So option B and C are correct.
Bromine is a weak oxidizing agent so it will oxidize the aldehydic group into carboxylic groups only. To convert the sucrose into saccharic acid, a strong oxidizing agent like concentrated sulphuric acid is needed.
Maltose is made up of two glucose units so hydrolysis by acid does not give us sucrose.
The correct statements are option B and option C.
Note:
Sucrose is a non-reducing sugar as it does not reduce Fehling or Tollens reagent indicating that the carbonyl group of both monosaccharides is involved in the linkage. It is optically active but does not undergo mutarotation. Its uses are-
-It is used as a coagulant agent.
-It is used to remove acidity from fruits.
-It is used for complete suspension of solid in a solution.
-It is used as a flavour enhancer.
Complete step by step answer:
The chemical formula of sucrose is ${C_{12}}{H_{22}}{O_{11}}$. Its structure is given as-

It occurs in nature Sucrose is called invert sugar because on hydrolysis with acid or enzyme, it gives equal parts of glucose and fructose which constitute the two monosaccharide units of sucrose. The reaction is given as-
${C_{12}}{H_{22}}{O_{11}} + {H_2}O \to \mathop {{C_6}{H_{12}}{O_6}}\limits_{D( + ) - {\text{glucose}}} + \mathop {{C_6}{H_{12}}{O_6}}\limits_{D( - ) - {\text{fructose}}} $
Sucrose is dextrorotatory with specific rotation \[ + {66^\circ }\] but it changes to laevorotatory due to formation of one mole of glucose and one mole of fructose which have specific rotations $ + {52^\circ }$ and $ - {92^\circ }$ respectively.
Net specific rotation is the average of specific rotation of glucose and fructose molecule as both monomers are present in equal amount so we can write-
$ \Rightarrow $ $\alpha = \dfrac{{52 + \left( { - 92} \right)}}{2}$
On solving, we get-
$ \Rightarrow $ $\alpha = - \dfrac{{40}}{2} = - {20^\circ }$
So option B and C are correct.
Bromine is a weak oxidizing agent so it will oxidize the aldehydic group into carboxylic groups only. To convert the sucrose into saccharic acid, a strong oxidizing agent like concentrated sulphuric acid is needed.
Maltose is made up of two glucose units so hydrolysis by acid does not give us sucrose.
The correct statements are option B and option C.
Note:
Sucrose is a non-reducing sugar as it does not reduce Fehling or Tollens reagent indicating that the carbonyl group of both monosaccharides is involved in the linkage. It is optically active but does not undergo mutarotation. Its uses are-
-It is used as a coagulant agent.
-It is used to remove acidity from fruits.
-It is used for complete suspension of solid in a solution.
-It is used as a flavour enhancer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




