
Formula for the carbide of silicon with its correct property is:
(A) $ S{i_2}C, $ very hard and used as abrasive
(B) $ SiC $ , very hard and used as abrasive
(C) $ S{i_2}C $ , very soft and used as lubricant
(D) $ SiC $ , very soft and used as lubricant
Answer
520.8k+ views
Hint :Carbide is a term used for compounds made up of metals and metalloids by ionic bonds or covalent bonds. In which carbide exists as $ {C_2}^{ - 2} $ which is a dianionic form. Hybridization of carbide ions is sp and it has linear shape. It has two carbon atoms with a lone pair on each atom.
Complete Step By Step Answer:
Carbides are mainly of four types: Ionic, Covalent, Interstitial, Transition metals carbide. Silicon forms covalent carbide because carbon and silicon have low electronegativity differences. Silicon carbide is generally known as carborundum. Structure $ SiC $ exists in many forms with hexagonal crystal structure as the most common.
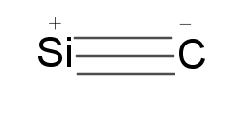
Silicon carbides are stable and inert compounds. It is very hard and used as an abrasive, its hardness is very close to diamond. They are good for making rocket engines sandpapers, heating elements etc.
Thus, option (B) $ SiC $ , very hard and used as abrasive is the correct choice.
Note :
$ SiC $ has a high thermal conductivity, temperature strength is very high along with properties like behaviour as semiconductors and low thermal expansion.
Silicon carbide dust has hazards to humans, creates irritation in eyes, skin and can even lead to lung cancer.
Complete Step By Step Answer:
Carbides are mainly of four types: Ionic, Covalent, Interstitial, Transition metals carbide. Silicon forms covalent carbide because carbon and silicon have low electronegativity differences. Silicon carbide is generally known as carborundum. Structure $ SiC $ exists in many forms with hexagonal crystal structure as the most common.
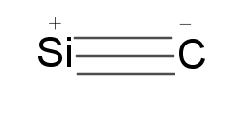
Silicon carbides are stable and inert compounds. It is very hard and used as an abrasive, its hardness is very close to diamond. They are good for making rocket engines sandpapers, heating elements etc.
Thus, option (B) $ SiC $ , very hard and used as abrasive is the correct choice.
Note :
$ SiC $ has a high thermal conductivity, temperature strength is very high along with properties like behaviour as semiconductors and low thermal expansion.
Silicon carbide dust has hazards to humans, creates irritation in eyes, skin and can even lead to lung cancer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




