
Fractional distillation works on the principle of:
(A) Varying boiling point
(B) Varying melting point
(C) Varying molar mass
(D) None of these
Answer
588k+ views
Hint: Fractional distillation is used to separate the two or more liquids that are miscible with each other. It is a special type of distillation. The process involves the heating of the liquid mixture. The liquid on heating boils and converts to vapours to attain the vapour pressure equal to the atmospheric pressure escape from the mixture. These vapours are then condensed to get the purified component.
Complete step by step solution:
Fractional distillation is a type of distillation. The process involves the separation of miscible liquids. The process involves the distillation and then followed by the condensation of the mixture into its separate components. The separation takes place when the mixture of liquids is heated to entrain temperature. At a particular temperature, the fractions of the mixture start to vaporize.
The basic principle of fractional distillation is that the different liquids boil at their boiling point at different temperatures. The boiling point is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure. The substance which has the low boiling point requires the less temperature to convert into the vapour. This component easily escapes from the mixture and the condenser condenses the vapour.
For example, gasoline and another chemical which are produced from crude oil can be separated by fractional distillation. Crude oil is first heated until it evaporates. The different fractions of the crude oil condense at the different temperature ranges. One of the fractions of crude oil is a hydrocarbon with a comparable number of atoms. The fractions might be naphtha, gasoline, kerosene, fuel oil, refinery gas, etc.
The simple fractional distillation assembly is as shown below:
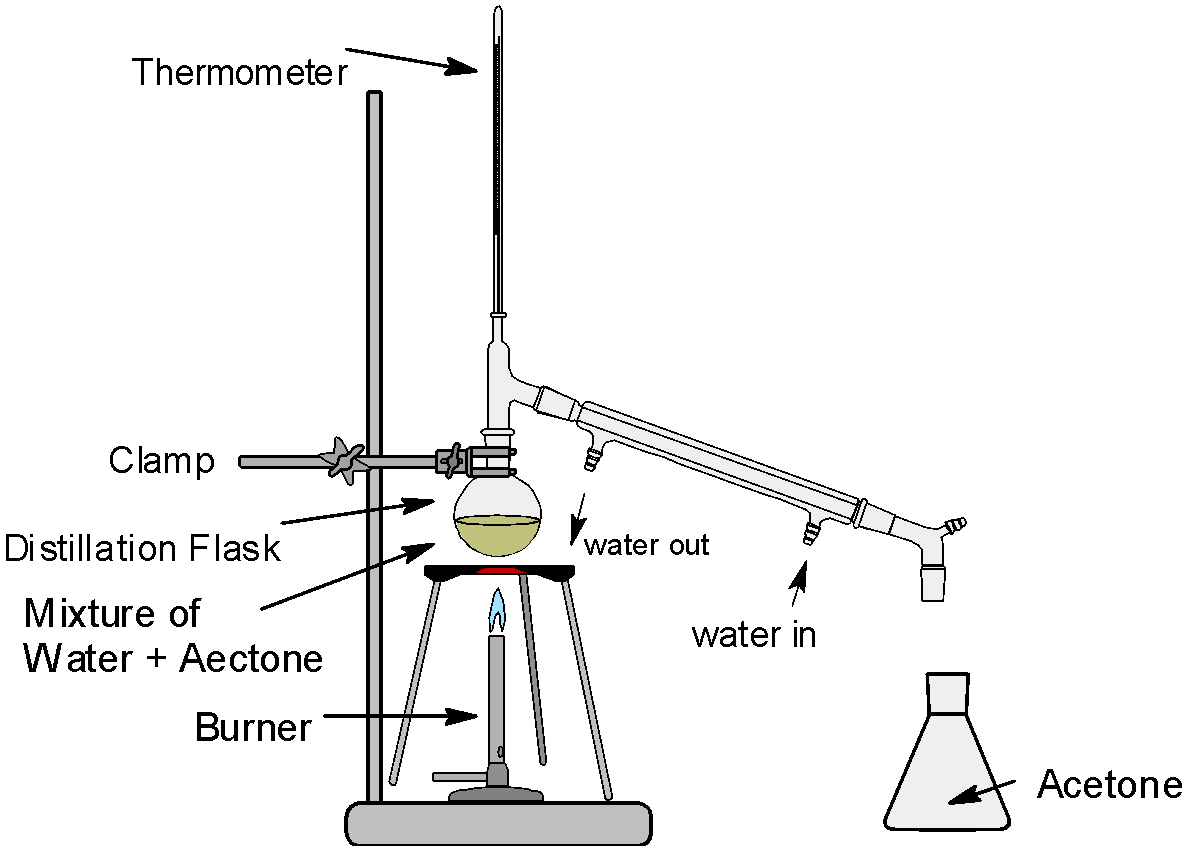
The acetone and water mixture is separated by the fractional distillation. The boiling point of acetone is \[\text{5}{{\text{6}}^{\text{0}}}\text{C}\] and the boiling point of water is\[{{100}^{\text{0}}}\text{C}\]. Thus the acetone is separated first followed by the water.
Fractional distillation works on the principle of varying boiling points.
Hence, (A) is the correct option.
NOTE: the water-ethanol cannot be separated by the fractional distillation. The ethanol has the boiling point \[{{78.1}^{\text{0}}}\text{C}\]and water boils at the\[{{100}^{\text{0}}}\text{C}\]. Since ethanol and water are a constant boiling mixture in which the composition of vapour is the same as that of the liquid. This is called the azeotrope.
Complete step by step solution:
Fractional distillation is a type of distillation. The process involves the separation of miscible liquids. The process involves the distillation and then followed by the condensation of the mixture into its separate components. The separation takes place when the mixture of liquids is heated to entrain temperature. At a particular temperature, the fractions of the mixture start to vaporize.
The basic principle of fractional distillation is that the different liquids boil at their boiling point at different temperatures. The boiling point is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure. The substance which has the low boiling point requires the less temperature to convert into the vapour. This component easily escapes from the mixture and the condenser condenses the vapour.
For example, gasoline and another chemical which are produced from crude oil can be separated by fractional distillation. Crude oil is first heated until it evaporates. The different fractions of the crude oil condense at the different temperature ranges. One of the fractions of crude oil is a hydrocarbon with a comparable number of atoms. The fractions might be naphtha, gasoline, kerosene, fuel oil, refinery gas, etc.
The simple fractional distillation assembly is as shown below:

The acetone and water mixture is separated by the fractional distillation. The boiling point of acetone is \[\text{5}{{\text{6}}^{\text{0}}}\text{C}\] and the boiling point of water is\[{{100}^{\text{0}}}\text{C}\]. Thus the acetone is separated first followed by the water.
Fractional distillation works on the principle of varying boiling points.
Hence, (A) is the correct option.
NOTE: the water-ethanol cannot be separated by the fractional distillation. The ethanol has the boiling point \[{{78.1}^{\text{0}}}\text{C}\]and water boils at the\[{{100}^{\text{0}}}\text{C}\]. Since ethanol and water are a constant boiling mixture in which the composition of vapour is the same as that of the liquid. This is called the azeotrope.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




