
Fraunhofer lines in solar spectrum can be cited as an example of –
A) Stefan’s law
B) Kirchhoff’s law
C) William’s law
D) Planck's law
Answer
565.2k+ views
Hint: We need to understand the reasons for the Fraunhofer’s lines in the spectral lines from solar radiation. Then, we can relate the given laws to the phenomenon and thereby find the most suitable explanation for the Fraunhofer lines.
Complete step-by-step solution:
We know that the Fraunhofer lines in a spectrum of radiation are the dark lines within the spectrum. These dark lines or the Fraunhofer lines are the result of selective absorption of the colour of a particular wavelength by the particles present in the path of the radiation.
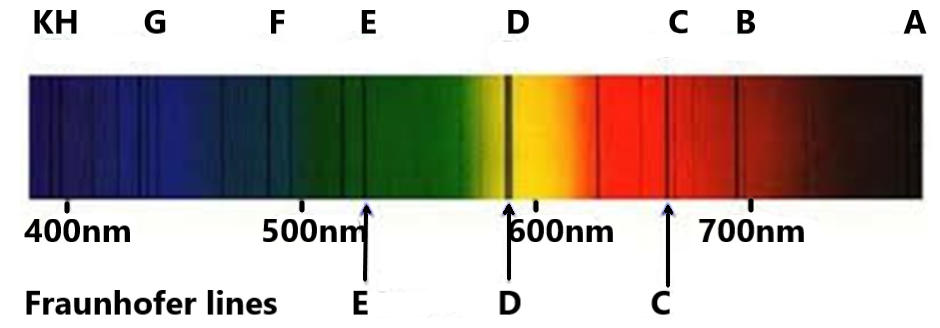
Now, let us discuss the different laws given to us and identify the one which describes the Fraunhofer lines.
Stefan’s law: It states that the rate of energy radiated from a body is directly proportional to its emissivity, surface area and the fourth power of the temperature difference with the surrounding.
Kirchhoff’s law: It states that a hot matter, whether solid, liquid or gas will give a continuous spectrum under high pressures. At low pressures the matter gives an emission line spectrum.
William’s law: It states that in a spectrum of a radiation, one can see dark lines or some missing colours due to the selective absorption of the wavelength by particles in the path of the radiation.
Planck's law: It gives the mathematical relationship on the spectral-energy distribution of a radiation emitted by a body.
Comparing the four laws, we understand the Fraunhofer lines are best described by William's law.
The correct answer is option C.
Note: The Kirchhoff’s law has an extension which says that hot matter can give a continuous spectrum with dark lines when the radiation is passed through a low-pressure gas. But the law doesn’t give an explanation for these dark lines in the spectrum.
Complete step-by-step solution:
We know that the Fraunhofer lines in a spectrum of radiation are the dark lines within the spectrum. These dark lines or the Fraunhofer lines are the result of selective absorption of the colour of a particular wavelength by the particles present in the path of the radiation.

Now, let us discuss the different laws given to us and identify the one which describes the Fraunhofer lines.
Stefan’s law: It states that the rate of energy radiated from a body is directly proportional to its emissivity, surface area and the fourth power of the temperature difference with the surrounding.
Kirchhoff’s law: It states that a hot matter, whether solid, liquid or gas will give a continuous spectrum under high pressures. At low pressures the matter gives an emission line spectrum.
William’s law: It states that in a spectrum of a radiation, one can see dark lines or some missing colours due to the selective absorption of the wavelength by particles in the path of the radiation.
Planck's law: It gives the mathematical relationship on the spectral-energy distribution of a radiation emitted by a body.
Comparing the four laws, we understand the Fraunhofer lines are best described by William's law.
The correct answer is option C.
Note: The Kirchhoff’s law has an extension which says that hot matter can give a continuous spectrum with dark lines when the radiation is passed through a low-pressure gas. But the law doesn’t give an explanation for these dark lines in the spectrum.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




