
Fructose is an example of:
(A) Ketohexose
(B) Aldohexose
(C) Ketopentose
(D) Aldopentose
Answer
502.2k+ views
Hint :Carbohydrates are sugar molecules. It contains mainly carbon, hydrogen and oxygen atoms. Carbohydrates are mainly classified into three types. The monosaccharides are one of the types and contain one monomer unit. Fructose is a monosaccharide with six carbon atoms containing a ketone group.
Complete Step By Step Answer:
Chemical compounds are different types. Carbohydrates are the essential nutrients to the body. Carbohydrates mainly contain carbon, hydrogen and oxygen atoms. These carbohydrates are sugar molecules. The general molecular formula of carbohydrates is $ {C_x}{\left( {{H_2}O} \right)_y} $ , where x and y are the number of carbon atoms and y is the sum of the number of hydrogen and oxygen atoms.
Simply, carbohydrates are known as hydrates of carbon. Carbohydrates are classified into three types based on the number of monosaccharides units.
Fructose is an example of monosaccharide with the molecular formula of $ {C_6}{H_{12}}{O_6} $ , it contains hydroxyl group and ketone group. Thus, it has a ketone group with six carbon atoms.
The structure of fructose is
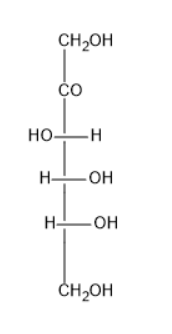
From the above structure, it was known that fructose contains five hydroxyl groups and one ketone group. Ketone is a carbonyl group.
Thus, fructose is an example of ketohexose.
Option C is the correct one.
Note :
Monosaccharides contain only one simple unit and further do not undergo hydrolysis to form a simple sugar. Oligosaccharides contain monosaccharides from two to ten in number. Polysaccharides contain a large number of monosaccharide units and on hydrolysis forms multiple monosaccharide units.
Complete Step By Step Answer:
Chemical compounds are different types. Carbohydrates are the essential nutrients to the body. Carbohydrates mainly contain carbon, hydrogen and oxygen atoms. These carbohydrates are sugar molecules. The general molecular formula of carbohydrates is $ {C_x}{\left( {{H_2}O} \right)_y} $ , where x and y are the number of carbon atoms and y is the sum of the number of hydrogen and oxygen atoms.
Simply, carbohydrates are known as hydrates of carbon. Carbohydrates are classified into three types based on the number of monosaccharides units.
Fructose is an example of monosaccharide with the molecular formula of $ {C_6}{H_{12}}{O_6} $ , it contains hydroxyl group and ketone group. Thus, it has a ketone group with six carbon atoms.
The structure of fructose is

From the above structure, it was known that fructose contains five hydroxyl groups and one ketone group. Ketone is a carbonyl group.
Thus, fructose is an example of ketohexose.
Option C is the correct one.
Note :
Monosaccharides contain only one simple unit and further do not undergo hydrolysis to form a simple sugar. Oligosaccharides contain monosaccharides from two to ten in number. Polysaccharides contain a large number of monosaccharide units and on hydrolysis forms multiple monosaccharide units.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




