
Fructose reduces Tollen’s reagent due to:
A. asymmetric carbons
B. primary alcoholic groups
C. secondary alcoholic groups
D. enolisation of fructose followed by conversion to aldehyde by base
Answer
544.5k+ views
Hint: According to the question, first we will check Fructose is from which species and the species of fructose can reduce Tollen’s reagent or not. And then try to know why Fructose is able to reduce Tollen’s reagent. . Aldehydes are oxidised easily but ketones do not easily oxidise.
Complete step by step solution:
We know that only aldehydes can reduce Tollen’s reagent. But we know Fructose is a Ketone, so fructose is kept in an aqueous solution and enolised. And then converted into aldehyde in basic medium or by base.
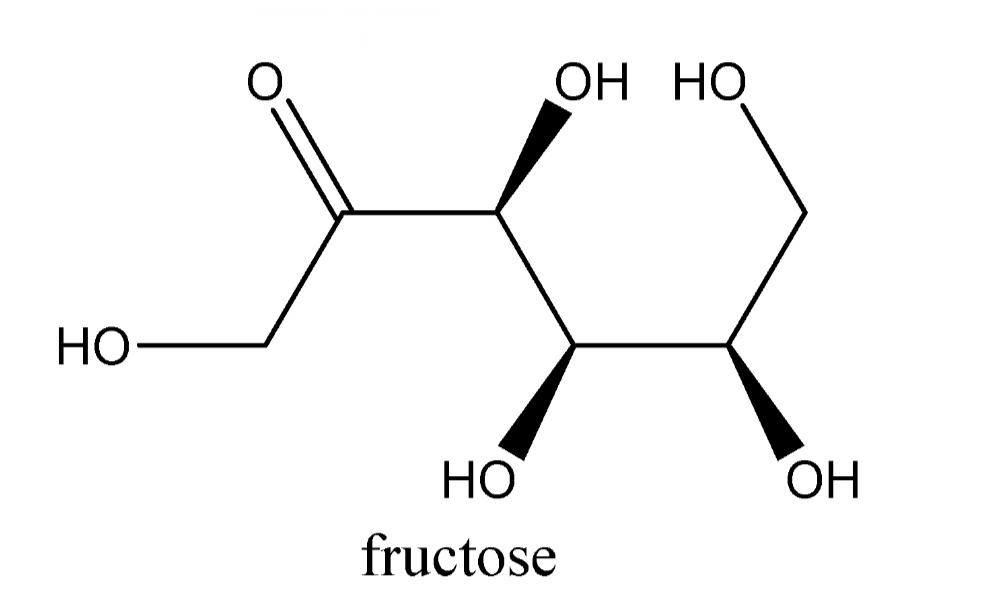
The reaction of aldehydes with Tollen’s reagent is an oxidation reaction. Aldehydes are oxidised easily but ketones do not. So, ketone cannot reduce Tollen’s reagent and we know Fructose is a ketone.
That’s why there is a need to convert Fructose into aldehyde by keeping it in a base for enolisation. Then after conversion Fructose starts reducing Tollen’s reagent. All aldehydes generally reduces tollens reagent thus fructose also Tollens reagent.
Finally we conclude that fructose reduces Tollen’s reagent due to enolisation of fructose followed by conversion to aldehyde by base.
Hence the correct option is D.
Note:
In other ways, in the presence of alkali, fructose is converted into a mixture of mannose and glucose showing enolisation. Glucose then reduces Tollen’s reagent. We can also reduce alpha hydroxy ketones by Tollen’s reagent.
Complete step by step solution:
We know that only aldehydes can reduce Tollen’s reagent. But we know Fructose is a Ketone, so fructose is kept in an aqueous solution and enolised. And then converted into aldehyde in basic medium or by base.

The reaction of aldehydes with Tollen’s reagent is an oxidation reaction. Aldehydes are oxidised easily but ketones do not. So, ketone cannot reduce Tollen’s reagent and we know Fructose is a ketone.
That’s why there is a need to convert Fructose into aldehyde by keeping it in a base for enolisation. Then after conversion Fructose starts reducing Tollen’s reagent. All aldehydes generally reduces tollens reagent thus fructose also Tollens reagent.
Finally we conclude that fructose reduces Tollen’s reagent due to enolisation of fructose followed by conversion to aldehyde by base.
Hence the correct option is D.
Note:
In other ways, in the presence of alkali, fructose is converted into a mixture of mannose and glucose showing enolisation. Glucose then reduces Tollen’s reagent. We can also reduce alpha hydroxy ketones by Tollen’s reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




