
How many gauche conformations are possible for n-butane?
A. 2
B. 3
C. 4
D. 1
Answer
582.6k+ views
Hint: Conformational isomerism is a kind of stereoisomerism. In confirmation isomerism, the isomers can be inter-convertible by rotations about single bonds. Among the different conformations, Gauche conformation has less steric strain when compared to eclipsed conformation. Less strain means low internal energy and high stability.
Complete step by step solution:
- We have to find how many gauche conformations are possible by n-butane.
- n-butane contains 4 carbon atoms.
- The below representations are called Newman’s conformations of n-butane.
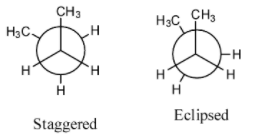
- By rotating one of the carbon atoms by keeping another carbon fixed we will get different conformations of n-butane.
- The following are the possible conformations of n-butane by rotating one of the carbon by keeping another carbon atom fixed.
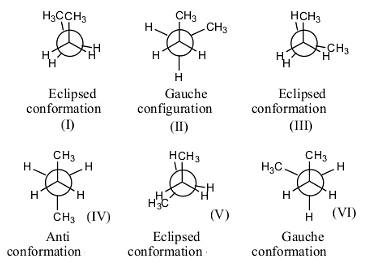
- There are a total of 6 confirmations possible for n-butane.
- Out of those 6, three confirmations are Eclipsed (I, III and V), two confirmations are gauche (II and VI), and one anti conformation (IV).
- Gauche conformation has less strain when compared to eclipsed conformation.
- In eclipsed conformation, the atoms are going to face high repulsion because of the overlapping of one atom with another.
- In n-butane, there are two gauche conformations with less strain.
- Therefore the number of gauche conformations possible for n-butane is 2.
So, the correct option is A.
Note: In Gauche conformation the two atoms or groups have a dihedral angle of more than 0 and less than 120. A conformation in a molecule has one or more gauche connections then it can be called as gauche conformation and gauche conformations are stable than eclipsed conformations.
Complete step by step solution:
- We have to find how many gauche conformations are possible by n-butane.
- n-butane contains 4 carbon atoms.
- The below representations are called Newman’s conformations of n-butane.

- By rotating one of the carbon atoms by keeping another carbon fixed we will get different conformations of n-butane.
- The following are the possible conformations of n-butane by rotating one of the carbon by keeping another carbon atom fixed.

- There are a total of 6 confirmations possible for n-butane.
- Out of those 6, three confirmations are Eclipsed (I, III and V), two confirmations are gauche (II and VI), and one anti conformation (IV).
- Gauche conformation has less strain when compared to eclipsed conformation.
- In eclipsed conformation, the atoms are going to face high repulsion because of the overlapping of one atom with another.
- In n-butane, there are two gauche conformations with less strain.
- Therefore the number of gauche conformations possible for n-butane is 2.
So, the correct option is A.
Note: In Gauche conformation the two atoms or groups have a dihedral angle of more than 0 and less than 120. A conformation in a molecule has one or more gauche connections then it can be called as gauche conformation and gauche conformations are stable than eclipsed conformations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




