
Geometry of $B{F_3}$ is
A: Trigonal planar
B: Pentagonal
C: Linear
D: Tetrahedral
Answer
596.4k+ views
Hint: VSEPR theory is used to predict the geometry of individual molecules from the number of electron pairs surrounding the central atom. VSEPR theory is valence shell electron pair repulsion theory.
Complete step by step solution:
$B{F_3}$ is boron trifluoride. As we know VSEPR theory is used to predict the shape of compounds. According to this theory a compound will have trigonal planar geometry if it contains $3$ bond pair and $0$ lone pair. This kind of geometry has $s{p^2}$ hybridization. Pentagonal geometry if it contains $5$bond pair and $0$ lone pair. This kind of geometry will have $s{p^3}d$hybridization. Linear geometry if it contains $2$ bond pair and $0$ lone pair. Tetrahedral geometry if it contains $4$ bond pair and $0$ lone pair. This kind of geometry will have $s{p^3}$ hybridization. Structure of $B{F_3}$contains $3$ bond pair and $0$ lone pair. This means it will have trigonal planar geometry. As shown below.
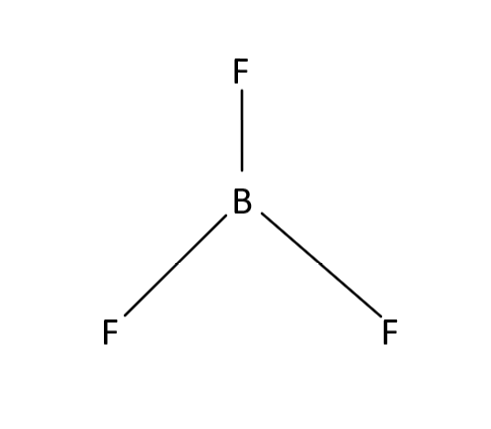
So our answer to this question is option A that is Trigonal planar.
Additional information: VSEPR theory was unable to explain the exact shape of molecules in many cases. Taking direction of electron pairs doesn’t seem to be very rational.
To explain the concept of shapes of molecules clearly hybridization was introduced. It involves intermixing of two or more atomic orbitals of slightly different energies but of the same atom so that a redistribution of energy takes place between them resulting in the formation of an equal number of new orbitals which are called hybrid orbitals which will have the same energy, size and shape.
For molecules or ions having regular geometry, change in electronegativity of the central atom or the surrounding atom has no effect on the bond angle.
The actual structure is in between all the contributing structures and is called resonance hybrid. The different individual structures are called resonating structures or canonical forms. This phenomenon is called resonance.
Note: Different compounds have different geometry according to the number of lone pair and bond pair. So remember the number of bond pairs and lone pairs for which different geometries of compounds are formed.
Complete step by step solution:
$B{F_3}$ is boron trifluoride. As we know VSEPR theory is used to predict the shape of compounds. According to this theory a compound will have trigonal planar geometry if it contains $3$ bond pair and $0$ lone pair. This kind of geometry has $s{p^2}$ hybridization. Pentagonal geometry if it contains $5$bond pair and $0$ lone pair. This kind of geometry will have $s{p^3}d$hybridization. Linear geometry if it contains $2$ bond pair and $0$ lone pair. Tetrahedral geometry if it contains $4$ bond pair and $0$ lone pair. This kind of geometry will have $s{p^3}$ hybridization. Structure of $B{F_3}$contains $3$ bond pair and $0$ lone pair. This means it will have trigonal planar geometry. As shown below.

So our answer to this question is option A that is Trigonal planar.
Additional information: VSEPR theory was unable to explain the exact shape of molecules in many cases. Taking direction of electron pairs doesn’t seem to be very rational.
To explain the concept of shapes of molecules clearly hybridization was introduced. It involves intermixing of two or more atomic orbitals of slightly different energies but of the same atom so that a redistribution of energy takes place between them resulting in the formation of an equal number of new orbitals which are called hybrid orbitals which will have the same energy, size and shape.
For molecules or ions having regular geometry, change in electronegativity of the central atom or the surrounding atom has no effect on the bond angle.
The actual structure is in between all the contributing structures and is called resonance hybrid. The different individual structures are called resonating structures or canonical forms. This phenomenon is called resonance.
Note: Different compounds have different geometry according to the number of lone pair and bond pair. So remember the number of bond pairs and lone pairs for which different geometries of compounds are formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




