
What is the geometry of the chromate ion?
A. Tetrahedral
B. Octahedral
C. Trigonal planar
D. Linear
Answer
607.8k+ views
Hint- In order to find the geometry of a given compound first we will understand the compound chromate ion, and then we will proceed further by using its formula and IUPAC name to make the structure of the chromate ion.
Complete answer:
Chromates are the salts of chromic acid which contain the chromate anion with chemical formula $CrO_4^{2 - }$ and usually have an intense yellow color.
Chromate is the oxoanion that occurs from chromic acid treatment of the protons. This is also called chrome oxoanion or inorganic divalent anions. The most commonly used chromate salts are sodium and potassium salts.
IUPAC name – dioxide (dioxo) chromium
Chromate Structure $CrO_4^{2 - }$
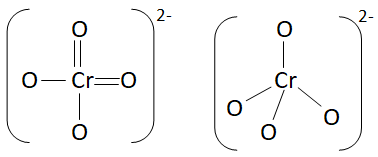
From the figure it is clear that the geometry is tetrahedral.
Hence, the geometry of chromate ions is tetrahedral.
So, the correct answer is option A.
Additional information- Chromates and dichromates are used in chromium plating to protect metals from corrosion and improve the adhesion of paints. Chromate and dichromate salts of heavy metals, lanthanides, and alkaline earth metals are only somewhat water soluble and are often used as pigments. The lead-containing dye chrome yellow was used for a very long time until its use was prohibited by environmental legislation. Once used in a redox chemical reaction as oxidizing agents or titrants, chromates and dichromates convert into trivalent chromium, the salts of which usually have a strikingly different blue-green hue.
Note- Through focusing solely on the number of electron pairs around the central atom, we may use the VSEPR model to determine the structure of certain polyatomic molecules and ions, ignoring any other valence electrons present. Sodium and potassium salts of chromate are highly corrosive and used in enamels, finishing leather and for rust proofing metals. It is mainly used as an inhibitor of corrosion, as a primer, as a decorative finish, or to maintain electrical conductance.
Complete answer:
Chromates are the salts of chromic acid which contain the chromate anion with chemical formula $CrO_4^{2 - }$ and usually have an intense yellow color.
Chromate is the oxoanion that occurs from chromic acid treatment of the protons. This is also called chrome oxoanion or inorganic divalent anions. The most commonly used chromate salts are sodium and potassium salts.
IUPAC name – dioxide (dioxo) chromium
Chromate Structure $CrO_4^{2 - }$

From the figure it is clear that the geometry is tetrahedral.
Hence, the geometry of chromate ions is tetrahedral.
So, the correct answer is option A.
Additional information- Chromates and dichromates are used in chromium plating to protect metals from corrosion and improve the adhesion of paints. Chromate and dichromate salts of heavy metals, lanthanides, and alkaline earth metals are only somewhat water soluble and are often used as pigments. The lead-containing dye chrome yellow was used for a very long time until its use was prohibited by environmental legislation. Once used in a redox chemical reaction as oxidizing agents or titrants, chromates and dichromates convert into trivalent chromium, the salts of which usually have a strikingly different blue-green hue.
Note- Through focusing solely on the number of electron pairs around the central atom, we may use the VSEPR model to determine the structure of certain polyatomic molecules and ions, ignoring any other valence electrons present. Sodium and potassium salts of chromate are highly corrosive and used in enamels, finishing leather and for rust proofing metals. It is mainly used as an inhibitor of corrosion, as a primer, as a decorative finish, or to maintain electrical conductance.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




