
Give one chemical test to distinguish between aniline and benzylamine.
Answer
577.5k+ views
Hint:. In this chemical test a weak acid is used. This acid is used to make diazonium salts from amines. It is pale blue. It decomposes slowly at room temperature—and more rapidly at elevated temperatures—to nitric acid and nitric oxide.
Complete step by step answer:
-We should first know about our chemicals that have to be tested.
-So, benzylamine is an organic chemical compound with the condensed structural formula ${{C}_{6}}{{H}_{5}}C{{H}_{2}}N{{H}_{2}}$. We should note that benzylamine is a colourless liquid with a characteristic anime-like odour with strong alkaline properties. It is solvable in water. Benzyl amine is mainly used in chemical synthesis, and for production of pesticides, pharmaceutical substances.
-Aniline is an organic compound with the formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. These compounds are said to be toxic and also known to be one of the classes of aromatic amines. These are used in a wide variety of industries and are known to possess all the characteristics of an aromatic compound.
-Now, we will test benzylamine and aniline one by one.
-We can distinguish aniline and benzylamine by their reactions with the help of nitrous acid.
So, first, we will do a reaction of benzylamine with nitrous acid.
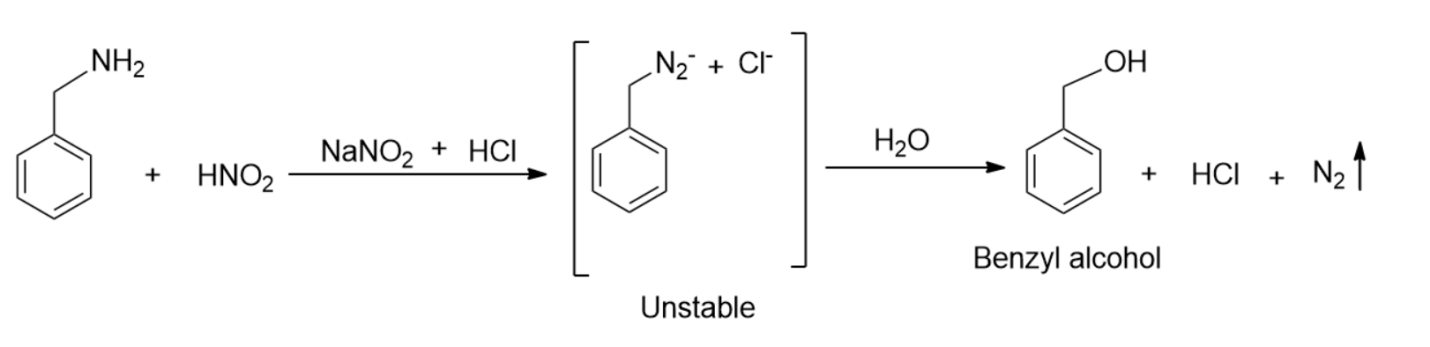
-We will notice in the above reaction that benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
-Now, we will react to aniline with nitrous acid.
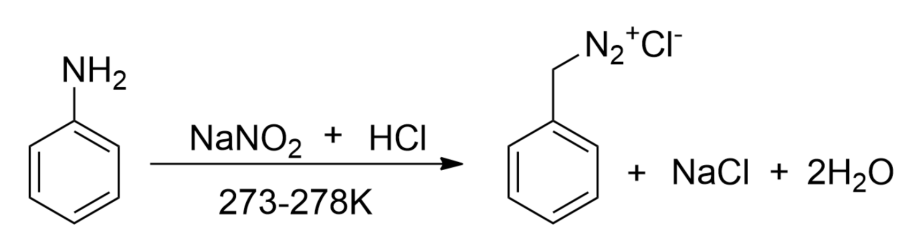
-On the other hand, we will notice that aniline reacts with nitrous acid at a low temperature to form stable diazonium salt. Thus, nitrogen gas is not evolved.
Note: -We should be careful in handling chemicals.
-Benzyl amine is corrosive. If benzylamine gets in contact with skin, it causes burns. Its vapours are highly irritating to mucous membranes. It will cause eye burns. If swallowed, its ingestion will be harmful.
-If we get contacted with aniline it will cause dizziness, headaches, irregular heartbeat, convulsions, coma, and death may also occur. Direct contact with aniline can also produce skin and eye irritation. Long-term exposure to lower levels of aniline may cause symptoms similar to those experienced in acute high-level exposure.
Complete step by step answer:
-We should first know about our chemicals that have to be tested.
-So, benzylamine is an organic chemical compound with the condensed structural formula ${{C}_{6}}{{H}_{5}}C{{H}_{2}}N{{H}_{2}}$. We should note that benzylamine is a colourless liquid with a characteristic anime-like odour with strong alkaline properties. It is solvable in water. Benzyl amine is mainly used in chemical synthesis, and for production of pesticides, pharmaceutical substances.
-Aniline is an organic compound with the formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. These compounds are said to be toxic and also known to be one of the classes of aromatic amines. These are used in a wide variety of industries and are known to possess all the characteristics of an aromatic compound.
-Now, we will test benzylamine and aniline one by one.
-We can distinguish aniline and benzylamine by their reactions with the help of nitrous acid.
So, first, we will do a reaction of benzylamine with nitrous acid.

-We will notice in the above reaction that benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
-Now, we will react to aniline with nitrous acid.

-On the other hand, we will notice that aniline reacts with nitrous acid at a low temperature to form stable diazonium salt. Thus, nitrogen gas is not evolved.
Note: -We should be careful in handling chemicals.
-Benzyl amine is corrosive. If benzylamine gets in contact with skin, it causes burns. Its vapours are highly irritating to mucous membranes. It will cause eye burns. If swallowed, its ingestion will be harmful.
-If we get contacted with aniline it will cause dizziness, headaches, irregular heartbeat, convulsions, coma, and death may also occur. Direct contact with aniline can also produce skin and eye irritation. Long-term exposure to lower levels of aniline may cause symptoms similar to those experienced in acute high-level exposure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




