
Give one example of a condensation polymer.
Answer
581.7k+ views
Hint: First, we have to understand the condensation polymers. Condensation polymers are formed when the small monomeric units react with each other to form the larger polymeric units with a loss of water molecule.
Complete answer:
Let’s begin with the basic concept of polymerization. Polymerization is the process in which small monomer molecules react and form long-chain polymeric molecules. There are two types of polymerization: addition and condensation polymerization. When smaller monomers units react with each other to form polymer without any loss of water molecule is called as addition polymerization. While condensation polymers form with a loss of water molecules.
Condensation polymers form slowly as compared to the additional polymers because condensation polymer requires heat. It is a step-growth polymerization. The condensation polymers contain functional groups like amine, alcohol, carboxylic acid.
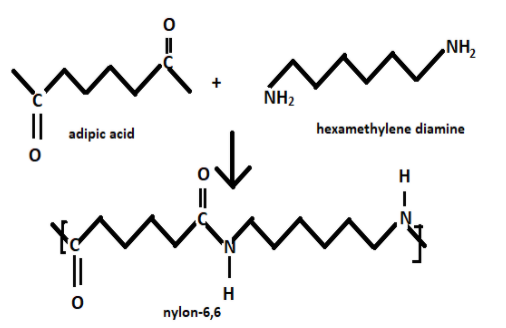
Nylon and polyesters are examples of the condensation polymers. When the monomers of hexamethylene diamine and adipic acid react with each other it gives the final product i.e., Nylon-6,6 and by-product water molecules. Nylon- 6,6 has 12 carbon units and it is used in making bristles for the brushes, sheets and in textile industries. It has greater resistance towards water, chemicals and heat.
Polyester forms by the reaction between glycol and dibasic acid. The by-product is also water that is mostly removed.
Note:
Nylon-6,6 is a polyamide that is formed by the condensation and step-growth polymerization. Remember nylon-6,6 is a light weight material so it is used in making parachutes. Do not confuse condensation polymerization with the elimination reaction as in condensation reaction, water molecules are eliminated.
Complete answer:
Let’s begin with the basic concept of polymerization. Polymerization is the process in which small monomer molecules react and form long-chain polymeric molecules. There are two types of polymerization: addition and condensation polymerization. When smaller monomers units react with each other to form polymer without any loss of water molecule is called as addition polymerization. While condensation polymers form with a loss of water molecules.
Condensation polymers form slowly as compared to the additional polymers because condensation polymer requires heat. It is a step-growth polymerization. The condensation polymers contain functional groups like amine, alcohol, carboxylic acid.

Nylon and polyesters are examples of the condensation polymers. When the monomers of hexamethylene diamine and adipic acid react with each other it gives the final product i.e., Nylon-6,6 and by-product water molecules. Nylon- 6,6 has 12 carbon units and it is used in making bristles for the brushes, sheets and in textile industries. It has greater resistance towards water, chemicals and heat.
Polyester forms by the reaction between glycol and dibasic acid. The by-product is also water that is mostly removed.
Note:
Nylon-6,6 is a polyamide that is formed by the condensation and step-growth polymerization. Remember nylon-6,6 is a light weight material so it is used in making parachutes. Do not confuse condensation polymerization with the elimination reaction as in condensation reaction, water molecules are eliminated.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




