
Give only reaction for the following
1. Acetaldehyde to formaldehyde.
2. Formaldehyde to acetaldehyde.
3. Acetaldehyde to crotonic acid.
Answer
528.9k+ views
Hint :We know that acetaldehyde is an organic compound which is considered as an aldehyde. It has the chemical formula as $ C{{H}_{3}}CHO $ . The other name of acetaldehyde is MeCHO. It is a flammable liquid. Whereas formaldehyde is an organic compound with a chemical formula $ HCHO $ . It contains only one atom of carbon in it.
Complete Step By Step Answer:
To convert the aldehyde compound which contains two carbon atoms is known as acetaldehyde into the aldehyde compound which contains one carbon atom called formaldehyde. So first to convert the acetaldehyde into formaldehyde we will first oxidise the acetaldehyde into the acetic acid.
Then on heating the acetic acid with ammonia we get the compound called as acetamide which on further reacting with the bromine and the potassium hydroxide gives methyl amine. Then this methyl amine with the nitrous acid gives methyl alcohol which on oxidation gives formaldehyde. The following reactions are given as:
1. Acetaldehyde to formaldehyde:
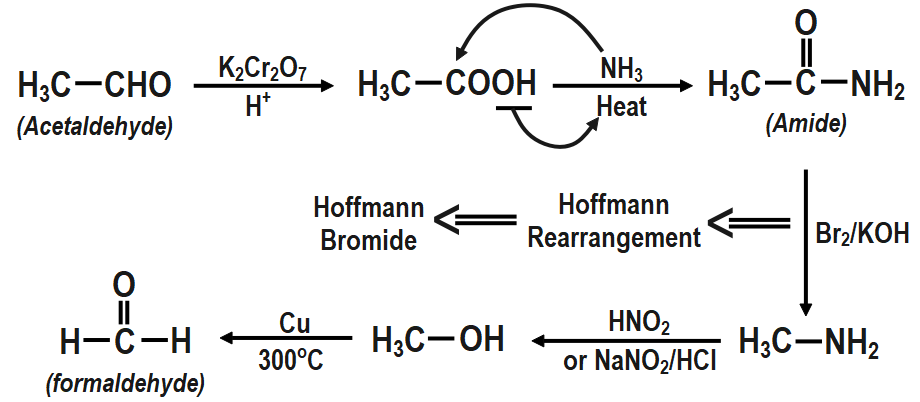
2. Formaldehyde to acetaldehyde:
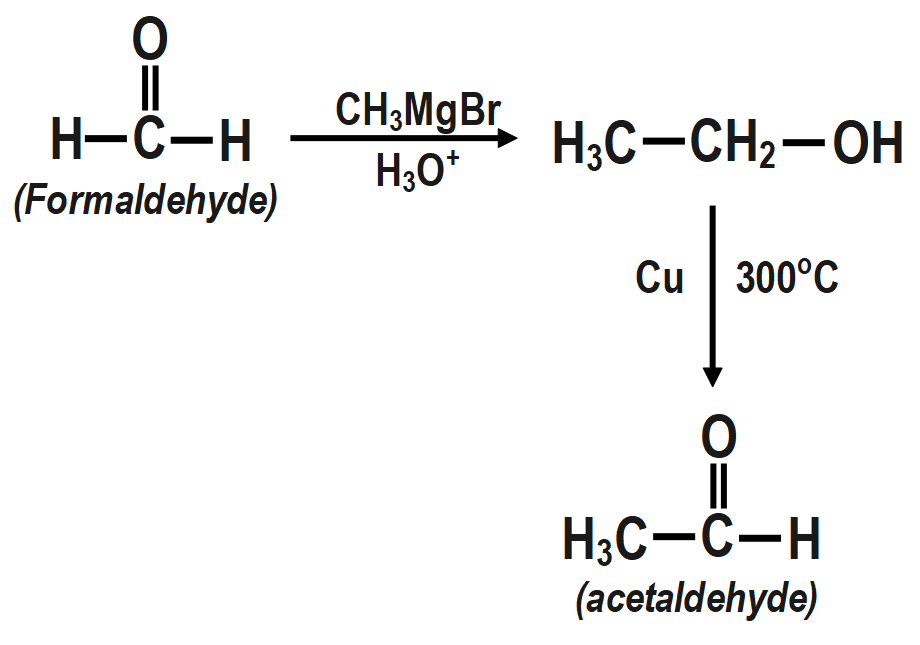
3. Acetaldehyde to crotonic acid.
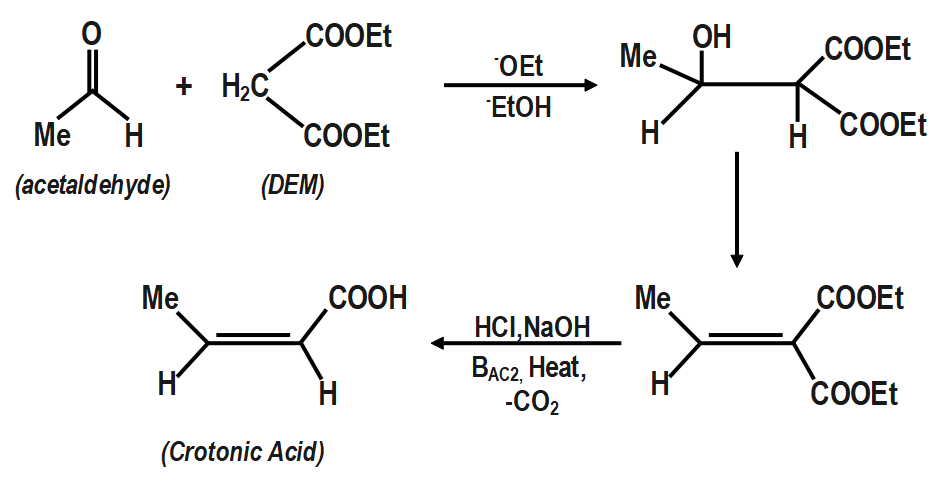
Note :
Note that the step where we converted the acetamide into methyl amine by reacting it with bromine and potassium hydroxide the reaction followed is known as Hoffmann bromamide reaction. In this reaction the degradation of the amine takes place leading to form the primary amine. The primary amine which is formed generally has the one less carbon atom in that of the amide. In the mechanism of this reaction usually a strong alkali is used.
Complete Step By Step Answer:
To convert the aldehyde compound which contains two carbon atoms is known as acetaldehyde into the aldehyde compound which contains one carbon atom called formaldehyde. So first to convert the acetaldehyde into formaldehyde we will first oxidise the acetaldehyde into the acetic acid.
Then on heating the acetic acid with ammonia we get the compound called as acetamide which on further reacting with the bromine and the potassium hydroxide gives methyl amine. Then this methyl amine with the nitrous acid gives methyl alcohol which on oxidation gives formaldehyde. The following reactions are given as:
1. Acetaldehyde to formaldehyde:

2. Formaldehyde to acetaldehyde:

3. Acetaldehyde to crotonic acid.

Note :
Note that the step where we converted the acetamide into methyl amine by reacting it with bromine and potassium hydroxide the reaction followed is known as Hoffmann bromamide reaction. In this reaction the degradation of the amine takes place leading to form the primary amine. The primary amine which is formed generally has the one less carbon atom in that of the amide. In the mechanism of this reaction usually a strong alkali is used.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




