
Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Answer
521.4k+ views
Hint:Strong bonds are responsible for higher boiling point, the molecule with higher boiling point must have stronger bonds which needs more energy to break.
Complete step by step answer:
Ethanol will undergo intermolecular hydrogen bonding due to the presence of $OH$ group or due to the presence of hydrogen attached to the electronegative oxygen atom.
Intermolecular hydrogen bondings are strong and hence require a large amount of energy to break these hydrogen bonds.
In a solution of water and ethanol, hydrogen bonding is the strongest intermolecular force between molecules. Hydrogen bonding occurs when the partially negative oxygen end of the molecules is attracted to the partially positive hydrogen eng of the molecule.
That is why the boiling point of ethanol is higher.
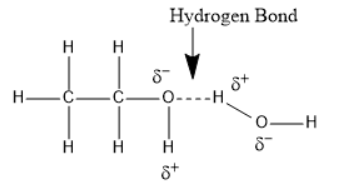
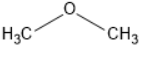
Methoxymethane doesn’t undergo hydrogen bonding and hence it requires less amount of energy to break bonds
That is why the boiling point of Methoxymethane is lower than that of ethanol.
Therefore, Intermolecular hydrogen bonding is strong and hence requires a large amount of energy to break these hydrogen bonds due to which its boiling point is higher than that of Methoxymethane.
Note:
Please see that hydrogen is attached directly to one of the most electronegative elements, causing hydrogen to acquire a significant amount of positive charge.
You can also notice that alcohols(in this case ethanol) have the greater solubility in water, this is because the $OH$ group present in the alcohols form hydrogen bonding with water and this intermolecular hydrogen bonding is very strong.
Complete step by step answer:
Ethanol will undergo intermolecular hydrogen bonding due to the presence of $OH$ group or due to the presence of hydrogen attached to the electronegative oxygen atom.
Intermolecular hydrogen bondings are strong and hence require a large amount of energy to break these hydrogen bonds.
In a solution of water and ethanol, hydrogen bonding is the strongest intermolecular force between molecules. Hydrogen bonding occurs when the partially negative oxygen end of the molecules is attracted to the partially positive hydrogen eng of the molecule.
That is why the boiling point of ethanol is higher.

Methoxymethane doesn’t undergo hydrogen bonding and hence it requires less amount of energy to break bonds
That is why the boiling point of Methoxymethane is lower than that of ethanol.

Therefore, Intermolecular hydrogen bonding is strong and hence requires a large amount of energy to break these hydrogen bonds due to which its boiling point is higher than that of Methoxymethane.
Note:
Please see that hydrogen is attached directly to one of the most electronegative elements, causing hydrogen to acquire a significant amount of positive charge.
You can also notice that alcohols(in this case ethanol) have the greater solubility in water, this is because the $OH$ group present in the alcohols form hydrogen bonding with water and this intermolecular hydrogen bonding is very strong.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




