
Give reason: Why is ammonia highly soluble in water?
Answer
558.3k+ views
Hint: \[{{N}}{{{H}}_{{3}}}\] is a polar molecule. In \[{{N}}{{{H}}_{{3}}}\] nitrogen is more electronegative than hydrogen, thus dipole moments of hydrogen are pointing to the nitrogen. The lone pair on nitrogen pushes the hydrogen away, making the dipole moments point in a common direction. This makes \[{{N}}{{{H}}_{{3}}}\] polar, this nature contributes to the solubility.
Complete step by step answer:
Ammonia, also called azane, is obtained when hydrogen and nitrogen react. Its molecular formula is \[{{N}}{{{H}}_{{3}}}\]. It is lighter than air and is widely used as a fertilizer. It is also used in the manufacturing of explosives and in the production of soda ash and in the Ostwald process to get nitric acid. Ammonia is behaving as a weak base since it combines with acids to form salts. It is also showing weak acidic qualities and, is therefore, called amphoteric. The acidity of ammonia enables it to form amides with some alkali metals and alkaline earth metals.
It is highly soluble in water. The high solubility of ammonia is due to the presence of lone pairs at the nitrogen. It is attracted to hydrogen in the water molecule hydrogen bonds. The presence of hydrogen bonding between two molecules means that the molecules are polar. In case of solubility, dissolves like i.e., polar molecules will dissolve in polar solvents. This means the molecules will be soluble in a polar solvent such as water. In other words, the polarity of these molecules indicates that they dissolve in water.
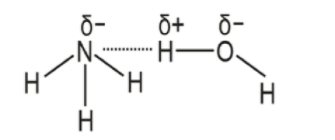
Hence, it is due to the lone pair atom that attracts hydrogen in water molecules.
Additional Information:
Solubility is the maximum amount of solute that can be dissolved in a known quantity of solvent at a certain temperature.
Note: There are two types of H bonds, called Intermolecular Hydrogen Bonding, when hydrogen bonding takes in different molecules it is called intermolecular hydrogen bonding and Intramolecular Hydrogen Bonding, when the hydrogen bonding which takes place within the same molecule itself is called intramolecular hydrogen bonding. The hydrogen bond is formed between the electronegative atom of one group with the hydrogen atoms of the other group.
Complete step by step answer:
Ammonia, also called azane, is obtained when hydrogen and nitrogen react. Its molecular formula is \[{{N}}{{{H}}_{{3}}}\]. It is lighter than air and is widely used as a fertilizer. It is also used in the manufacturing of explosives and in the production of soda ash and in the Ostwald process to get nitric acid. Ammonia is behaving as a weak base since it combines with acids to form salts. It is also showing weak acidic qualities and, is therefore, called amphoteric. The acidity of ammonia enables it to form amides with some alkali metals and alkaline earth metals.
It is highly soluble in water. The high solubility of ammonia is due to the presence of lone pairs at the nitrogen. It is attracted to hydrogen in the water molecule hydrogen bonds. The presence of hydrogen bonding between two molecules means that the molecules are polar. In case of solubility, dissolves like i.e., polar molecules will dissolve in polar solvents. This means the molecules will be soluble in a polar solvent such as water. In other words, the polarity of these molecules indicates that they dissolve in water.

Hence, it is due to the lone pair atom that attracts hydrogen in water molecules.
Additional Information:
Solubility is the maximum amount of solute that can be dissolved in a known quantity of solvent at a certain temperature.
Note: There are two types of H bonds, called Intermolecular Hydrogen Bonding, when hydrogen bonding takes in different molecules it is called intermolecular hydrogen bonding and Intramolecular Hydrogen Bonding, when the hydrogen bonding which takes place within the same molecule itself is called intramolecular hydrogen bonding. The hydrogen bond is formed between the electronegative atom of one group with the hydrogen atoms of the other group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




