
Give reasons for the following -
(A) Aniline does not undergo Friedel – Crafts reaction.
(B) ${{(C{{H}_{3}})}_{2}}NH$ is more basic than ${{(C{{H}_{3}})}_{3}}N$ in an aqueous solution.
(C) Primary amines have higher boiling point than tertiary amines.
Answer
529.9k+ views
Hint: (A) Aniline is a strong lewis base and the catalyst used ($AlC{{l}_{3}}$) is a strong lewis acid.
(B) Consider inductive effect, solvation effect and steric effect. The molecule favoured in more than one effect will be the answer.
(C) Consider the difference in extent of H bonding in both compounds.
Complete-step- by- step answer:
(A) The Friedel – Crafts reaction is an organic reaction in which an electrophilic aromatic substitution occurs which causes attachment of substituents to the aromatic ring.
For this reaction, $AlC{{l}_{3}}\text{ or }FeC{{l}_{3}}$ is used as a catalyst. The role of the catalyst is to extract chlorine atoms from the reactant and promote the chlorination of aromatic molecules so that HCl is removed from the aromatic compound.
For example, for Friedel – Crafts alkylation, the mechanism is as shown below.
\[R-Cl+FeC{{l}_{3}}\to {{R}^{+}}+FeC{{l}_{4}}^{-}\]
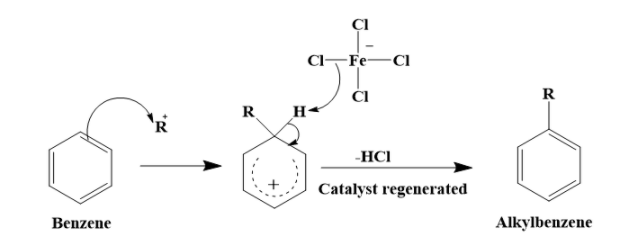 As you can see, the catalyst plays a very important role in the reaction. It generates the alkyl carbocation that combines with the benzene in the next step to form the alkyl benzene.
As you can see, the catalyst plays a very important role in the reaction. It generates the alkyl carbocation that combines with the benzene in the next step to form the alkyl benzene.
For Aniline, the Friedel – Crafts reaction does not occur. Due to the lone pair of electrons on N, aniline is a strong lewis base. Also, the catalyst used ($AlC{{l}_{3}}$ ) is a very strong lewis acid.
So, an acid base reaction occurs between aniline and $AlC{{l}_{3}}$ leading to salt formation. The salt formed acts as a deactivating group and hence the electrophilic substitution cannot happen.
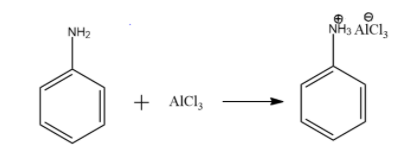
Hence, aniline does not undergo Friedel – Crafts reaction.
(B) ${{(C{{H}_{3}})}_{2}}NH$ is a secondary amine and ${{(C{{H}_{3}})}_{3}}N$ is a tertiary amine. We have to understand why a secondary amine is more basic than the tertiary amine. The basicity of an amine is determined by the availability of the lone pair on the N atom.
Let us first consider the inductive effect. According to the inductive effect, the tertiary amine
will be more stable as it has more alkyl groups.
As it is an aqueous solution, it is very important that we consider the solvation effect too. In aqueous medium, the stability of the ion formed will be determined by H bonding. As secondary amines have more H atoms, they will be more stable. Hence, if we consider the solvation effect only, the secondary amine is more stable.
If we consider the steric effect, the secondary amine will be more basic as it has less steric hindrance.
As there are 2 factors favouring the secondary amine, we can conclude that ${{(C{{H}_{3}})}_{2}}NH$ is more basic than ${{(C{{H}_{3}})}_{3}}N$ in an aqueous solution.
(C) We have to understand why Primary amines have higher boiling point than tertiary amines.
We know that primary amines have more H atoms than tertiary amines. This means that primary amines will have greater extent of intermolecular hydrogen bonding.
The greater the intermolecular bonding, the harder it will be to break the bonds. Therefore, it will take more energy to break bonds in primary amines than in tertiary amines.
Hence we can conclude that Primary amines have higher boiling point than tertiary amines.
Note: For part (B) you should be careful that you remember to consider all the 3 effects. You might get the wrong answer if you only consider one of the effects.
(B) Consider inductive effect, solvation effect and steric effect. The molecule favoured in more than one effect will be the answer.
(C) Consider the difference in extent of H bonding in both compounds.
Complete-step- by- step answer:
(A) The Friedel – Crafts reaction is an organic reaction in which an electrophilic aromatic substitution occurs which causes attachment of substituents to the aromatic ring.
For this reaction, $AlC{{l}_{3}}\text{ or }FeC{{l}_{3}}$ is used as a catalyst. The role of the catalyst is to extract chlorine atoms from the reactant and promote the chlorination of aromatic molecules so that HCl is removed from the aromatic compound.
For example, for Friedel – Crafts alkylation, the mechanism is as shown below.
\[R-Cl+FeC{{l}_{3}}\to {{R}^{+}}+FeC{{l}_{4}}^{-}\]

For Aniline, the Friedel – Crafts reaction does not occur. Due to the lone pair of electrons on N, aniline is a strong lewis base. Also, the catalyst used ($AlC{{l}_{3}}$ ) is a very strong lewis acid.
So, an acid base reaction occurs between aniline and $AlC{{l}_{3}}$ leading to salt formation. The salt formed acts as a deactivating group and hence the electrophilic substitution cannot happen.

Hence, aniline does not undergo Friedel – Crafts reaction.
(B) ${{(C{{H}_{3}})}_{2}}NH$ is a secondary amine and ${{(C{{H}_{3}})}_{3}}N$ is a tertiary amine. We have to understand why a secondary amine is more basic than the tertiary amine. The basicity of an amine is determined by the availability of the lone pair on the N atom.
Let us first consider the inductive effect. According to the inductive effect, the tertiary amine
will be more stable as it has more alkyl groups.
As it is an aqueous solution, it is very important that we consider the solvation effect too. In aqueous medium, the stability of the ion formed will be determined by H bonding. As secondary amines have more H atoms, they will be more stable. Hence, if we consider the solvation effect only, the secondary amine is more stable.
If we consider the steric effect, the secondary amine will be more basic as it has less steric hindrance.
As there are 2 factors favouring the secondary amine, we can conclude that ${{(C{{H}_{3}})}_{2}}NH$ is more basic than ${{(C{{H}_{3}})}_{3}}N$ in an aqueous solution.
(C) We have to understand why Primary amines have higher boiling point than tertiary amines.
We know that primary amines have more H atoms than tertiary amines. This means that primary amines will have greater extent of intermolecular hydrogen bonding.
The greater the intermolecular bonding, the harder it will be to break the bonds. Therefore, it will take more energy to break bonds in primary amines than in tertiary amines.
Hence we can conclude that Primary amines have higher boiling point than tertiary amines.
Note: For part (B) you should be careful that you remember to consider all the 3 effects. You might get the wrong answer if you only consider one of the effects.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




