
Give the allylic, vinylic or benzylic & common name of following compounds.

Answer
568.5k+ views
Hint:
Allylic halides are compounds containing halogen atoms bonded to \[s{p^3}\] hybridized C-atom next to carbon carbon double bond. Vinylic halides are compounds containing halogen atom bonded to \[s{p^2}\] hybridized C-atom of an aliphatic compound
Benzylic halides are compounds containing halogen atoms bonded to \[s{p^3}\] hybridized C-atoms next to aromatic rings.
Complete step by step answer:
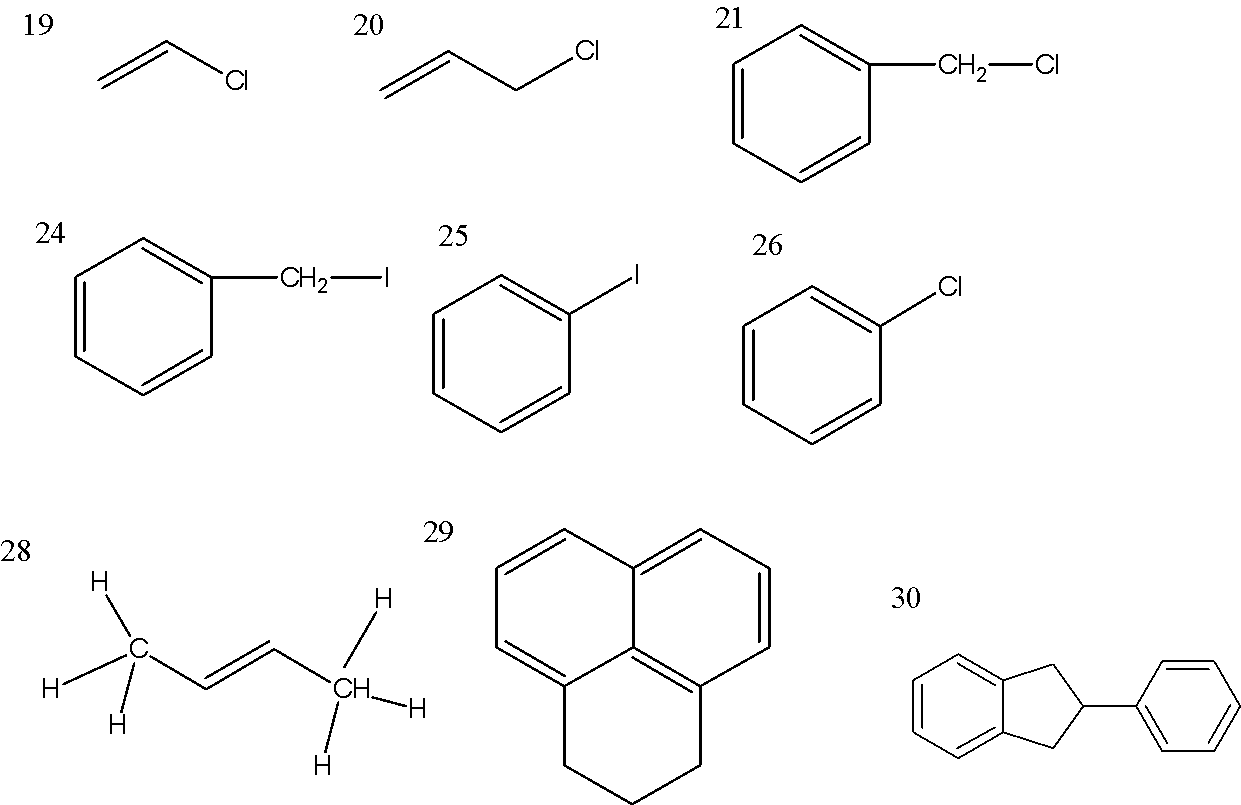
At 19.
As Cl is attach to \[s{p^2}\] hybridized C-atom of aliphatic compound so the name is vinyl chloride and the common name is chloroethene
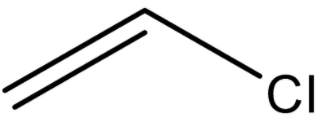
In 20.
As we can see the Chlorine attached to ${\text{s}}{{\text{p}}^{\text{3}}}$ dized C-atom of an aliphatic compound, so name is allyl chloride and the common name is 3-chloroprop-1-ene.
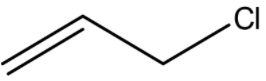
In 21.
As Cl is attached to \[s{p^3}\] hybridized C-atom next to aromatic ring, so the name is benzyl chloride and the common name is α-chlorotoluene.
In 24.
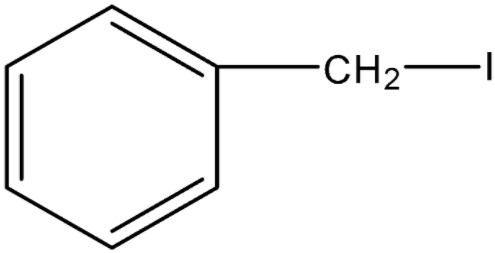
As Iodine is attached to \[s{p^3}\] hybridized C-atom next to the aromatic ring, so the name is benzyl iodide and the common name is α-iodotoluene.
In25.
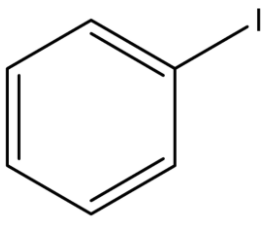
As iodine is directly attached to $sp^2$ hybridized C-atom of benzene ring. As iodine is directly attached to benzene so the name is iodobenzene and the common name is phenyl iodide.
At 26.
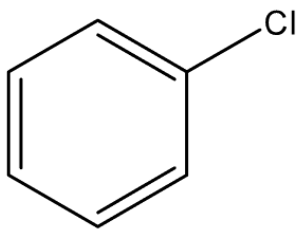
As Chlorine is directly attached to $sp^2$ hybridized C-atom of benzene ring. As chlorine is directly attached to benzene so the name is chlorobenzene and the common name is phenyl chloride.
In 28.
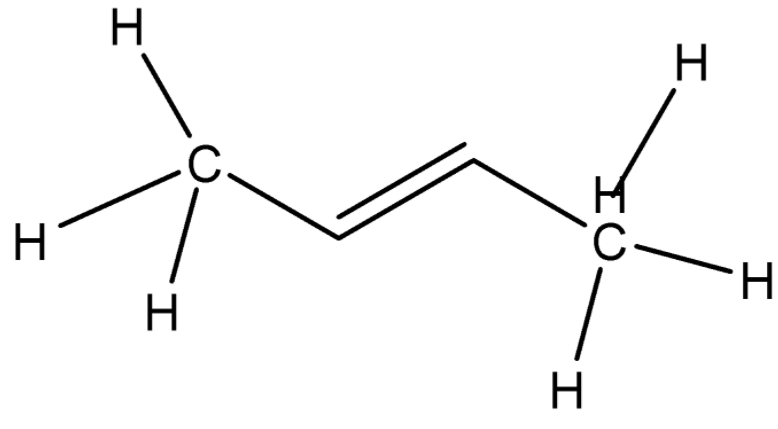
Here, the double bond is between C1 and C2 so it is an alkene. The name is 2-Butene.
At 29.
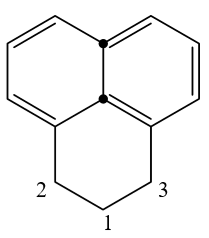
Here C2 and C3 are benzylic carbon and C1 is connected to both benzylic carbons.
In 30.
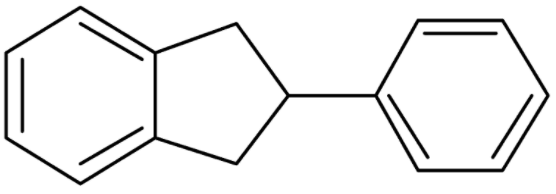
Additional information:
Allylic and benzylic compounds are more stable than vinylic compounds because of resonance in them which gives them stability, unlike vinylic which act as an electron withdrawing group.
Note:For naming a compound to be allylic, vinylic or benzylic it is recommended to check the hybridization of the attached carbon by which will be able to classify them properly.
Allylic halides are compounds containing halogen atoms bonded to \[s{p^3}\] hybridized C-atom next to carbon carbon double bond. Vinylic halides are compounds containing halogen atom bonded to \[s{p^2}\] hybridized C-atom of an aliphatic compound
Benzylic halides are compounds containing halogen atoms bonded to \[s{p^3}\] hybridized C-atoms next to aromatic rings.
Complete step by step answer:
At 19.

As Cl is attach to \[s{p^2}\] hybridized C-atom of aliphatic compound so the name is vinyl chloride and the common name is chloroethene
In 20.

As we can see the Chlorine attached to ${\text{s}}{{\text{p}}^{\text{3}}}$ dized C-atom of an aliphatic compound, so name is allyl chloride and the common name is 3-chloroprop-1-ene.
In 21.

As Cl is attached to \[s{p^3}\] hybridized C-atom next to aromatic ring, so the name is benzyl chloride and the common name is α-chlorotoluene.
In 24.

As Iodine is attached to \[s{p^3}\] hybridized C-atom next to the aromatic ring, so the name is benzyl iodide and the common name is α-iodotoluene.
In25.

As iodine is directly attached to $sp^2$ hybridized C-atom of benzene ring. As iodine is directly attached to benzene so the name is iodobenzene and the common name is phenyl iodide.
At 26.

As Chlorine is directly attached to $sp^2$ hybridized C-atom of benzene ring. As chlorine is directly attached to benzene so the name is chlorobenzene and the common name is phenyl chloride.
In 28.

Here, the double bond is between C1 and C2 so it is an alkene. The name is 2-Butene.
At 29.

Here C2 and C3 are benzylic carbon and C1 is connected to both benzylic carbons.
In 30.

Additional information:
Allylic and benzylic compounds are more stable than vinylic compounds because of resonance in them which gives them stability, unlike vinylic which act as an electron withdrawing group.
Note:For naming a compound to be allylic, vinylic or benzylic it is recommended to check the hybridization of the attached carbon by which will be able to classify them properly.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




