
Give the chemical reaction for the preparation of nitrile rubber. Also mention its two properties and two uses.
Answer
576.9k+ views
Hint:. as we know, nitrile rubber is a synthetic rubber so for its preparation we need to react to the copolymers of nitrile rubber, which are 1,4-butadiene and acrylonitrile.
Complete step by step answer:
We have been asked about the preparation of nitrile rubber, its properties and uses.
So, for that:
Nitrile rubber is also known as NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is unusual in being resistant to oil, fuel, and other chemicals.
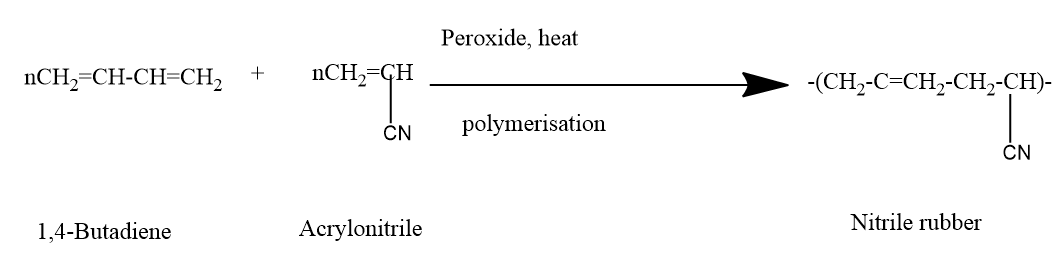
Now, for the preparation of nitrile rubber,
We know that Nitrile rubber is also called nitrile-butadiene rubber is an oil-resistant synthetic rubber produced from a copolymer of acrylonitrile and butadiene. Its main applications are in fuel hoses, gaskets, rollers, and other products in which oil resistance is required.
Now, the properties of nitrile rubber,
- It Possesses a high tensile strength $( >10N/m{{m}^ {2}}) $,
- Good resistance to mineral oils, vegetable oils, benzene/petrol, ordinary diluted acids and alkaline.
Now, the uses of nitrile rubber:
- It is used in the manufacturing of disposable non-latex gloves, automotive transmission belts, gaskets, synthetic leather etc.
- It can also be used in the preparation of adhesives and as a pigment binder.
Note: Nitrile gloves are therefore more puncture-resistant than natural rubber gloves, especially if the latter are degraded by exposure to chemicals or ozone. Nitrile rubber is less likely to cause an allergic reaction than natural rubber.
Complete step by step answer:
We have been asked about the preparation of nitrile rubber, its properties and uses.
So, for that:
Nitrile rubber is also known as NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is unusual in being resistant to oil, fuel, and other chemicals.
Now, for the preparation of nitrile rubber,
We know that Nitrile rubber is also called nitrile-butadiene rubber is an oil-resistant synthetic rubber produced from a copolymer of acrylonitrile and butadiene. Its main applications are in fuel hoses, gaskets, rollers, and other products in which oil resistance is required.

Now, the properties of nitrile rubber,
- It Possesses a high tensile strength $( >10N/m{{m}^ {2}}) $,
- Good resistance to mineral oils, vegetable oils, benzene/petrol, ordinary diluted acids and alkaline.
Now, the uses of nitrile rubber:
- It is used in the manufacturing of disposable non-latex gloves, automotive transmission belts, gaskets, synthetic leather etc.
- It can also be used in the preparation of adhesives and as a pigment binder.
Note: Nitrile gloves are therefore more puncture-resistant than natural rubber gloves, especially if the latter are degraded by exposure to chemicals or ozone. Nitrile rubber is less likely to cause an allergic reaction than natural rubber.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




