
Give the diazotization reaction of aniline. Also, give the chemical reaction involved in the preparation of red azo dye and light yellow azo dye.
Answer
588k+ views
Hint: The conversion of a primary amine into its diazonium salt is called diazotization. The temperature required for this reaction is in the range of 273K to 278K.
Complete step by step answer: Aromatic diazonium salt is generally prepared by adding a cold aqueous solution of sodium nitrite to the solution or suspension of a primary aromatic amine in acid at 273K to 278K. The general reaction is written as:
$ArN{H_2} + NaN{O_2} + 2HX\xrightarrow{{273K - 278K}}ArN_2^ + {X^ - } + NaX + 2{H_2}O$
Where Ar is an aryl group and X is halogen.
In the case of aniline diazotization reaction is written as:

Arenediazonium salts react with a highly reactive aromatic compound such as phenol and amines to form brightly colored azo compounds. This reaction is known as a coupling reaction.
The chemical reaction involved in the preparation of red azo dye:
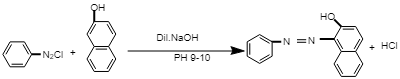
In this reaction, diazonium salt reacts with β-Naphthol in the presence of dil. NaOH at PH 9-10, i.e. in alkali medium to give β-Naphthol aniline dye, i.e. red azo dye.
The chemical reaction involved in the formation of yellow dye:
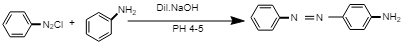
In this reaction, diazonium salt reacts with aniline in the presence of dil. NaOH at PH 4-5, i.e. in acidic medium to give p-Aminoazobenzene, i.e. yellow dye.
Note: The well-known indicator methyl orange used in acid-base titration is also prepared by a coupling reaction. So we can say diazonium salts are very good intermediates.
Complete step by step answer: Aromatic diazonium salt is generally prepared by adding a cold aqueous solution of sodium nitrite to the solution or suspension of a primary aromatic amine in acid at 273K to 278K. The general reaction is written as:
$ArN{H_2} + NaN{O_2} + 2HX\xrightarrow{{273K - 278K}}ArN_2^ + {X^ - } + NaX + 2{H_2}O$
Where Ar is an aryl group and X is halogen.
In the case of aniline diazotization reaction is written as:

Arenediazonium salts react with a highly reactive aromatic compound such as phenol and amines to form brightly colored azo compounds. This reaction is known as a coupling reaction.
The chemical reaction involved in the preparation of red azo dye:

In this reaction, diazonium salt reacts with β-Naphthol in the presence of dil. NaOH at PH 9-10, i.e. in alkali medium to give β-Naphthol aniline dye, i.e. red azo dye.
The chemical reaction involved in the formation of yellow dye:

In this reaction, diazonium salt reacts with aniline in the presence of dil. NaOH at PH 4-5, i.e. in acidic medium to give p-Aminoazobenzene, i.e. yellow dye.
Note: The well-known indicator methyl orange used in acid-base titration is also prepared by a coupling reaction. So we can say diazonium salts are very good intermediates.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




