
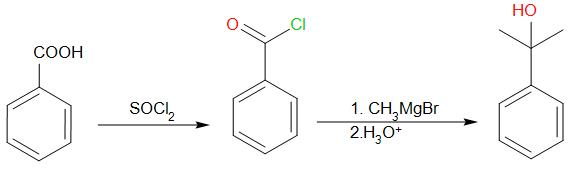
Give the following conversion: $PhCOOH \to PhC{\left( {C{H_3}} \right)_2}OH$
Answer
580.8k+ views
Hint: To answer this question, you need to recall reactions of carboxylic acids and their derivatives. The carbon atom in a carboxylic group is attached to two more electronegative oxygen atoms. As a result, the carbon atom is highly electrophilic and is susceptible to nucleophilic attack.
Complete step by step answer:
We know that acid chlorides are much more reactive than carboxylic acids. So, first we react benzoic acid with $SOC{l_2}$ to form benzoyl chloride.
Since we need to add two methyl groups to the carbonyl carbon, the best way to carry out that reaction is by using Grignard reagent. We react benzoyl chloride with two moles of Grignard reagent followed by exposure to acidic medium.
We will obtain the desired product.
Grignard reagents are important reagents in organic synthesis for creating new carbon-carbon bonds (increasing the chain size). They are prepared by reaction of organic halide with magnesium metal in anhydrous ether solvent. Since magnesium is a metal and is electropositive, the carbon atom attached to it has partial negative charge and acts as a nucleophile.
Pure Grignard reagents are extremely reactive compounds and react as carbanions. They are normally handled as solutions in anhydrous solvents such as diethyl ether or tetrahydrofuran which are relatively stable as long as water is excluded. In an ether solvent, Grignard reagent is invariably present as a complex with the magnesium atom connected to the two ether oxygen by coordination bonds which provides it extra stability.
Note:
Grignard reagents react with any compound that has a hydrogen atom attached to an electronegative atom. Any such compound is tremendously more acidic as compared to an alkane and therefore can decompose Grignard reagent. This is also a reason why Grignard reagent is not used in aqueous medium.
Complete step by step answer:
We know that acid chlorides are much more reactive than carboxylic acids. So, first we react benzoic acid with $SOC{l_2}$ to form benzoyl chloride.
Since we need to add two methyl groups to the carbonyl carbon, the best way to carry out that reaction is by using Grignard reagent. We react benzoyl chloride with two moles of Grignard reagent followed by exposure to acidic medium.
We will obtain the desired product.

Grignard reagents are important reagents in organic synthesis for creating new carbon-carbon bonds (increasing the chain size). They are prepared by reaction of organic halide with magnesium metal in anhydrous ether solvent. Since magnesium is a metal and is electropositive, the carbon atom attached to it has partial negative charge and acts as a nucleophile.
Pure Grignard reagents are extremely reactive compounds and react as carbanions. They are normally handled as solutions in anhydrous solvents such as diethyl ether or tetrahydrofuran which are relatively stable as long as water is excluded. In an ether solvent, Grignard reagent is invariably present as a complex with the magnesium atom connected to the two ether oxygen by coordination bonds which provides it extra stability.
Note:
Grignard reagents react with any compound that has a hydrogen atom attached to an electronegative atom. Any such compound is tremendously more acidic as compared to an alkane and therefore can decompose Grignard reagent. This is also a reason why Grignard reagent is not used in aqueous medium.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




