
Give the IUPAC name of compound given below:
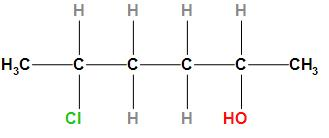
A, 2- Chloro -5-hydroxyhexane
B. 2- Hydroxy- 5- chlorohexane
C. 5- ChloroHexan-2-ol
D. 2- ChloroHexan-5-ol

Answer
563.4k+ views
Hint: The full form of IUPAC is the International Union of Pure and Applied Chemistry. In the chemical nomenclature, the IUPAC nomenclature in organic chemistry is a method used for the naming of organic chemical compounds. In the IUPAC system, the name of an organic compound consists of three parts:
Word root- It is the basic unit of the name. It depends upon the number of carbon atoms in the longest continuous carbon chain selected, called the parent chain. Depending upon the number of carbons in the chain the compound is assigned a word. For example, a chain containing ${C_2}$ carbons will be Eth.
The suffix- A suffix is added after the word root to indicate the nature of the carbon-carbon bond. For if the carbon chains contain a double bond then a suffix will be added as “ene”.
Prefix- The groups which are not regarded as functional but present in the carbon chain as substituents are written before the word root as a prefix. Such groups are fluorine, chlorine, nitro, etc.
Complete step by step answer:
Now we will discuss it in detail.
The structure of the given compound is
According to the IUPAC rule, the prefix (Chlorine) will be written first followed by the word root and primary suffix and secondary suffix. As the compound contains chlorine which will be regarded as substituents and will be written as a prefix. The compound contains six carbon atoms due to which it will be considered as hexane and the word root will be “hex”. Since it is an alkane compound so the primary suffix will be “ane” and the secondary suffix will “ol”. Therefore, the IUPAC name of the given compound is 5- Chlorohexan-2-ol.
So, the correct answer is Option C.
Note: In alcohols, the lower members are colourless volatile liquids which have a characteristic alcoholic odor and burning taste. Higher ones are solids. Methyl alcohols the first member of the alcohol group is a nerve poison and even its small doses cause blindness. Ethyl alcohol has a stimulant effect followed by depressant action on the central nervous system.
Word root- It is the basic unit of the name. It depends upon the number of carbon atoms in the longest continuous carbon chain selected, called the parent chain. Depending upon the number of carbons in the chain the compound is assigned a word. For example, a chain containing ${C_2}$ carbons will be Eth.
The suffix- A suffix is added after the word root to indicate the nature of the carbon-carbon bond. For if the carbon chains contain a double bond then a suffix will be added as “ene”.
Prefix- The groups which are not regarded as functional but present in the carbon chain as substituents are written before the word root as a prefix. Such groups are fluorine, chlorine, nitro, etc.
Complete step by step answer:
Now we will discuss it in detail.
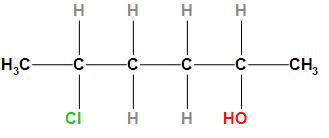
The structure of the given compound is

According to the IUPAC rule, the prefix (Chlorine) will be written first followed by the word root and primary suffix and secondary suffix. As the compound contains chlorine which will be regarded as substituents and will be written as a prefix. The compound contains six carbon atoms due to which it will be considered as hexane and the word root will be “hex”. Since it is an alkane compound so the primary suffix will be “ane” and the secondary suffix will “ol”. Therefore, the IUPAC name of the given compound is 5- Chlorohexan-2-ol.
So, the correct answer is Option C.
Note: In alcohols, the lower members are colourless volatile liquids which have a characteristic alcoholic odor and burning taste. Higher ones are solids. Methyl alcohols the first member of the alcohol group is a nerve poison and even its small doses cause blindness. Ethyl alcohol has a stimulant effect followed by depressant action on the central nervous system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




