
Give the Lewis structure for \[{\text{B}}{{\text{F}}_3}\] and \[{\text{Be}}{{\text{F}}_2}\].
Answer
568.8k+ views
Hint: In Lewis structure we represent both lone pair and bonded pairs of electrons. The lone pairs are the electrons generally present in the hybridised orbital.
Complete step by step answer:
The name of compound \[{\text{B}}{{\text{F}}_3}\] is boron trifluoride. The central atom here is boron. Boron belongs to group number 13 that is boron family. The atomic number of boron is 5 and it belongs to the second period. There are 3 valence electrons in case of boron. Fluorine belongs to group number 17 and is also known as chalcogens. The atomic number of fluorine is 9. There are seven valence electrons present in the outermost shell or valence shell of fluorine. It needs only one electron to fulfil its valency, due to which it acts as monovalent species. Monovalent species are those species which are attached through a single bond. all the three valence electron of boron are in bonded form with each fluorine and there is no lone pair of electrons on central atom the Lewis structure for boron trifluoride will be:
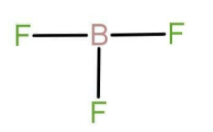
Now in the compound \[{\text{Be}}{{\text{F}}_2}\] the central atom is beryllium. Beryllium belongs to group number 2, that is it is an alkaline earth metal. The atomic number of boron is 4 and it belongs to the second period. There are 2 valence electrons in case of beryllium. It uses both of electrons in bond formation with fluorine and the Lewis structure is as follow:
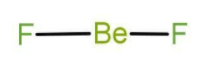
Note:
Both \[{\text{B}}{{\text{F}}_3}\] and \[{\text{Be}}{{\text{F}}_2}\] are known as Lewis acid because even after bond formation there octets are still incomplete and hence have the tendency to accept electron in order to get stability.
Complete step by step answer:
The name of compound \[{\text{B}}{{\text{F}}_3}\] is boron trifluoride. The central atom here is boron. Boron belongs to group number 13 that is boron family. The atomic number of boron is 5 and it belongs to the second period. There are 3 valence electrons in case of boron. Fluorine belongs to group number 17 and is also known as chalcogens. The atomic number of fluorine is 9. There are seven valence electrons present in the outermost shell or valence shell of fluorine. It needs only one electron to fulfil its valency, due to which it acts as monovalent species. Monovalent species are those species which are attached through a single bond. all the three valence electron of boron are in bonded form with each fluorine and there is no lone pair of electrons on central atom the Lewis structure for boron trifluoride will be:

Now in the compound \[{\text{Be}}{{\text{F}}_2}\] the central atom is beryllium. Beryllium belongs to group number 2, that is it is an alkaline earth metal. The atomic number of boron is 4 and it belongs to the second period. There are 2 valence electrons in case of beryllium. It uses both of electrons in bond formation with fluorine and the Lewis structure is as follow:

Note:
Both \[{\text{B}}{{\text{F}}_3}\] and \[{\text{Be}}{{\text{F}}_2}\] are known as Lewis acid because even after bond formation there octets are still incomplete and hence have the tendency to accept electron in order to get stability.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




