
Give the structural formula for Ethanal:
A. $C{H_3}CHO$
B. $C{H_3}C{H_2}O$
C. $C{H_2}CO$
D. HCHO
Answer
580.5k+ views
Hint: The aldehyde group is one of the functional groups in which the carbonyl carbon is present which is bonded with the oxygen atom by a double bond. It is also attached to one hydrogen atom and an alkyl group. The aldehyde compound is observed by the $- CHO$ group in the structure.
Complete step by step answer:
Structural formula: The structural formula of a chemical species is defined as the graphic presentation of all the atoms attached to form the compound in three-dimensional forms. In the structural formula, the bonding between the atoms is shown properly.
The structure of ethanal is shown below.
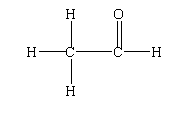
In this structure, two carbon atoms are present, therefore the parent chain is ethane. As the aldehyde functional group is present, the suffix is given by $- al$. Therefore the IUPAC name will be ethanal.
In ethanal, there are two carbons present. One is the carbonyl carbon and the other is the carbon present in the methyl group. The carbonyl carbon is attached to the oxygen by a double bond, with the hydrogen by a single bond and with the methyl group by a single bond.
Thus, the structural formula of ethanal is $C{H_3}CHO$.
Thus, the correct answer is option A.
Additional information: The parent chain in the aldehydic compound depends on the number of carbon atoms present in the compound. The compound ethanal is commonly known as acetaldehyde. The chemical formula of ethanal is also given as ${C_2}{H_4}O$.
Note:
There is a difference between structural formula and condensed structural formula. In condensed structural formula no chemical bonding is shown and the atoms are written in one line.
Complete step by step answer:
Structural formula: The structural formula of a chemical species is defined as the graphic presentation of all the atoms attached to form the compound in three-dimensional forms. In the structural formula, the bonding between the atoms is shown properly.
The structure of ethanal is shown below.

In this structure, two carbon atoms are present, therefore the parent chain is ethane. As the aldehyde functional group is present, the suffix is given by $- al$. Therefore the IUPAC name will be ethanal.
In ethanal, there are two carbons present. One is the carbonyl carbon and the other is the carbon present in the methyl group. The carbonyl carbon is attached to the oxygen by a double bond, with the hydrogen by a single bond and with the methyl group by a single bond.
Thus, the structural formula of ethanal is $C{H_3}CHO$.
Thus, the correct answer is option A.
Additional information: The parent chain in the aldehydic compound depends on the number of carbon atoms present in the compound. The compound ethanal is commonly known as acetaldehyde. The chemical formula of ethanal is also given as ${C_2}{H_4}O$.
Note:
There is a difference between structural formula and condensed structural formula. In condensed structural formula no chemical bonding is shown and the atoms are written in one line.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




