
Give the structure and IUPAC names of monohydric phenols of molecular formula, ${C_7}{H_8}O$.
Answer
594k+ views
Hint: Phenol is formed when a hydrogen atom in a benzene ring is replaced by a hydroxyl group. $ - OH$ group is called the hydroxyl group. Monohydric phenols are those which contain only one hydroxyl group.
Complete answer:In this question we have to find all the monohydric phenols of molecular formula ${C_7}{H_8}O$. We know phenols are aromatic compounds in which hydrogen is replaced by a hydroxyl group. In the benzene ring, there are only six carbons but in this question, seven carbons are given this means one hydrogen is replaced by a hydroxyl group and one hydrogen is replaced by some group containing carbon.
Due to extra carbon present in this formula, there will be an extra group other than the hydroxyl group (as we have to find monohydric phenols) and this extra group must contain carbon in it. And by interpreting the given formula there are three extra hydrogen atoms (one hydrogen from oh group four of benzene ring which is not replaced by any group so on subtracting these five hydrogen atoms from given eight hydrogen atoms we have extra three hydrogen atoms).
Now there is one carbon and three hydrogen atoms and one group to be formed so the only possibility is the methyl group formed by these four atoms. So we have three possible different structures with formula ${C_7}{H_8}O$ that is:
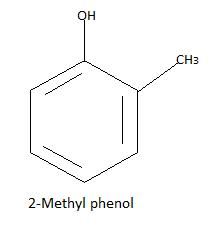
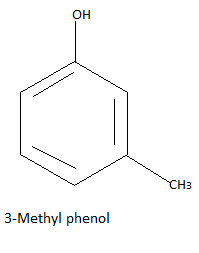
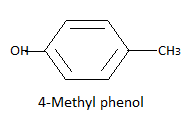
Note:
Like monohydric phenols there exist dihydric phenols and trihydric phenols. In dihydric phenols, two hydrogen atoms are replaced by a hydroxyl group and in trihydric phenol three hydrogen atoms are replaced by a hydroxyl group. These compounds are the major source of phenolic pollution in rivers and streams.
Complete answer:In this question we have to find all the monohydric phenols of molecular formula ${C_7}{H_8}O$. We know phenols are aromatic compounds in which hydrogen is replaced by a hydroxyl group. In the benzene ring, there are only six carbons but in this question, seven carbons are given this means one hydrogen is replaced by a hydroxyl group and one hydrogen is replaced by some group containing carbon.
Due to extra carbon present in this formula, there will be an extra group other than the hydroxyl group (as we have to find monohydric phenols) and this extra group must contain carbon in it. And by interpreting the given formula there are three extra hydrogen atoms (one hydrogen from oh group four of benzene ring which is not replaced by any group so on subtracting these five hydrogen atoms from given eight hydrogen atoms we have extra three hydrogen atoms).
Now there is one carbon and three hydrogen atoms and one group to be formed so the only possibility is the methyl group formed by these four atoms. So we have three possible different structures with formula ${C_7}{H_8}O$ that is:



Note:
Like monohydric phenols there exist dihydric phenols and trihydric phenols. In dihydric phenols, two hydrogen atoms are replaced by a hydroxyl group and in trihydric phenol three hydrogen atoms are replaced by a hydroxyl group. These compounds are the major source of phenolic pollution in rivers and streams.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




