
Given NaCl is an ionic compound. How is an ionic bond formed in NaCl?
Answer
571.5k+ views
Hint: We know that ionic compounds are usually compounds that have metal and non-metal. Ionic bonds hold the ions combined to give rise to ionic compounds. Ionic compounds are crystalline and the regular arrangement of ions and orderly way. Metal and non-metals could be present either in phase of solid, liquid (or) gas.
Complete step by step solution:
We know that ionic compounds are those compounds that are formed between a metal with a nonmetal when there is transfer of electrons. The bond formed in ionic compounds is known as ionic bonds. Ionic compounds exhibit the presence of positively charged ions termed as cations and negatively charged ions termed as anions. These compounds exist as solids in nature and show high boiling and melting points. When in molten or aqueous state, ionic compounds have the ability to conduct electricity.
Ionic bonding is the broad transfer of outermost electrons among atoms. In ionic bonds, we have to know that an electron is lost by metal and becomes positively charged cation whereas those electrons are accepted by nonmetals and become a negatively charged anion.
Sodium chloride is an ionic compound. The compound $NaCl$ comprises sodium and chlorine elements. Sodium is a metal that is present in group 1 (alkali metals) and chlorine is a nonmetal that is present in group 17 (halogens). The number of outermost electrons is one in sodium and seven in chlorine.
$NaCl$ is an ionic compound and so the formation takes place due to transfer of electrons from sodium to chlorine atom. Chlorine needs to gain one electron to attain its nearby gas stable electronic configuration and sodium needs to lose one electron to attain its stable electronic configuration. Therefore, sodium transfers one electron to chlorine. Chlorine gains one electron and sodium chloride is formed by ionic bond. The below image clearly shows how the bond is formed between the sodium and chlorine.
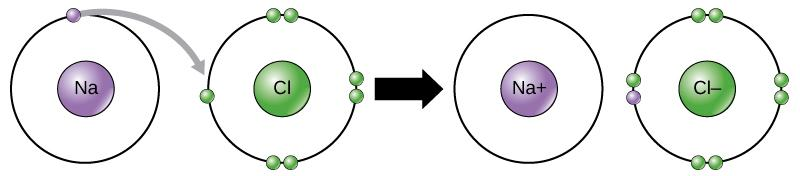
Note:We know that all ionic compounds contain cations as well as anions. The summation of the charges present in an ionic compound is always zero (overall). Ionic bonds hold the ions firmly leading to ionic compounds. Because of the existence of ionic bonds between them, they contain strong melting and boiling points.
Complete step by step solution:
We know that ionic compounds are those compounds that are formed between a metal with a nonmetal when there is transfer of electrons. The bond formed in ionic compounds is known as ionic bonds. Ionic compounds exhibit the presence of positively charged ions termed as cations and negatively charged ions termed as anions. These compounds exist as solids in nature and show high boiling and melting points. When in molten or aqueous state, ionic compounds have the ability to conduct electricity.
Ionic bonding is the broad transfer of outermost electrons among atoms. In ionic bonds, we have to know that an electron is lost by metal and becomes positively charged cation whereas those electrons are accepted by nonmetals and become a negatively charged anion.
Sodium chloride is an ionic compound. The compound $NaCl$ comprises sodium and chlorine elements. Sodium is a metal that is present in group 1 (alkali metals) and chlorine is a nonmetal that is present in group 17 (halogens). The number of outermost electrons is one in sodium and seven in chlorine.
$NaCl$ is an ionic compound and so the formation takes place due to transfer of electrons from sodium to chlorine atom. Chlorine needs to gain one electron to attain its nearby gas stable electronic configuration and sodium needs to lose one electron to attain its stable electronic configuration. Therefore, sodium transfers one electron to chlorine. Chlorine gains one electron and sodium chloride is formed by ionic bond. The below image clearly shows how the bond is formed between the sodium and chlorine.

Note:We know that all ionic compounds contain cations as well as anions. The summation of the charges present in an ionic compound is always zero (overall). Ionic bonds hold the ions firmly leading to ionic compounds. Because of the existence of ionic bonds between them, they contain strong melting and boiling points.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




