
Glucose is an example of
(A) Aldohexose
(B) Ketohexose
(C) Aldopentose
(D) ketopentose
Answer
584.7k+ views
Hint: Glucose consists of 6 carbon atoms and it also has an aldehyde group. The molecular formula of glucose is${{C}_{6}}{{H}_{12}}{{O}_{6}}$. Glucose is an organic compound.
Complete step by step solution:
We have to identify that glucose is an example of which of the given options. Glucose is an organic compound and a simple sugar. The general chemical formula for glucose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$.The structure of D and L form of glucose is mentioned below:
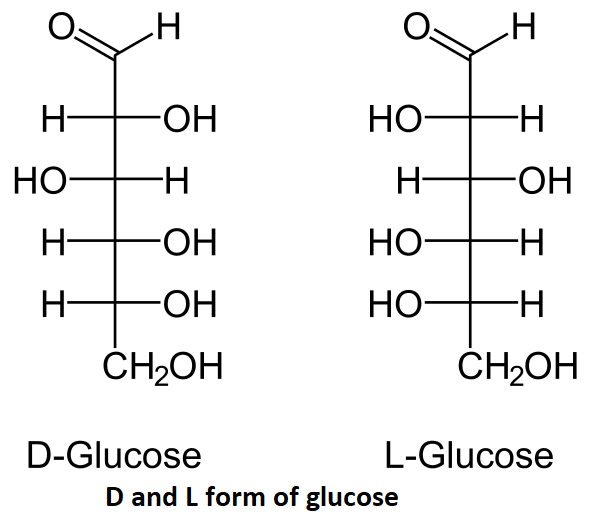
D-glucose stands for dextrorotatory glucose and L-glucose stands for levorotatory glucose, these two are the stereoisomers of glucose. From the above structure we can see that glucose contains 6 carbon atoms and it also contains 12 hydrogen atoms and 6 oxygen atoms. There is an aldehyde group present at the 6 carbon.
Aldohexose- it is a monosaccharide which contains both an aldehyde group and a six carbon chain. The aldehyde is known as aldose and the 6 carbon chain is known as hexose.
Ketohexose-it is also a monosaccharide but it has a keto group in its structure and also a carbon chain of 6 carbons.
As we already know that glucose contains 6 carbon atoms and an aldehyde group it is an Aldohexose.
Hence the correct answer is option (A) i.e. Glucose is an example of Aldohexose
Note: While solving such types of questions it is important to have prior knowledge about the structure of glucose. Glucose is also a monosaccharide which means that it is a simple reducing sugar, it is the most basic unit of carbohydrates.
Complete step by step solution:
We have to identify that glucose is an example of which of the given options. Glucose is an organic compound and a simple sugar. The general chemical formula for glucose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$.The structure of D and L form of glucose is mentioned below:

D-glucose stands for dextrorotatory glucose and L-glucose stands for levorotatory glucose, these two are the stereoisomers of glucose. From the above structure we can see that glucose contains 6 carbon atoms and it also contains 12 hydrogen atoms and 6 oxygen atoms. There is an aldehyde group present at the 6 carbon.
Aldohexose- it is a monosaccharide which contains both an aldehyde group and a six carbon chain. The aldehyde is known as aldose and the 6 carbon chain is known as hexose.
Ketohexose-it is also a monosaccharide but it has a keto group in its structure and also a carbon chain of 6 carbons.
As we already know that glucose contains 6 carbon atoms and an aldehyde group it is an Aldohexose.
Hence the correct answer is option (A) i.e. Glucose is an example of Aldohexose
Note: While solving such types of questions it is important to have prior knowledge about the structure of glucose. Glucose is also a monosaccharide which means that it is a simple reducing sugar, it is the most basic unit of carbohydrates.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




