
Glycerol does not contain __________ alcoholic group.
(A) $1^{\circ}$
(B) $2^{\circ}$
(C) $3^{\circ}$
(D) $1^{\circ}$ and $2^{\circ}$
Answer
573.6k+ views
Hint: The IUPAC name for glycerol is propane-$1,2,3$-triol, represented by the chemical formula $CH_2OH-CH_2OH-CH_2OH$. The degree of alcohol is determined by the degree of each carbon atom present in it. The degree of a carbon atom is the number of other carbon atoms surrounding it.
Complete step by step solution:
A carbon atom can be of three types - $1^{\circ}, 2^{\circ} , 3^{\circ}$.
$A 1^{\circ}$ carbon is a carbon atom that is not attached to any other carbon atom.
$A 2^{\circ}$ carbon is a carbon atom that is attached to one carbon atom and three other atoms.
$A 3^{\circ}$ carbon is a carbon atom that is attached to two other carbon atoms and another atom.
The structure of propane-1,2,3-triol can be represented as under:
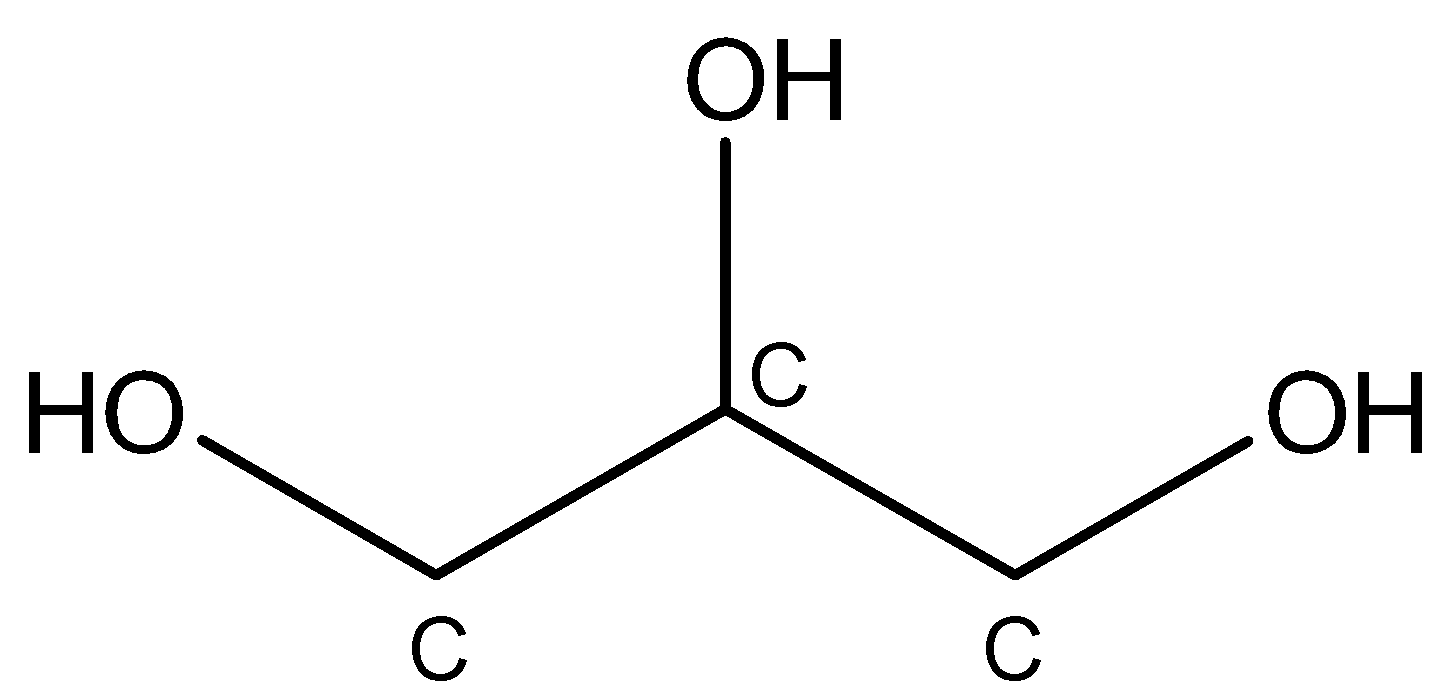
The first carbon atom is attached to the second carbon atom, two hydrogen atoms, and one $OH$ group. Since it is attached to only one carbon atom, the first carbon atom is of $1^{\circ}$.
The second carbon atom is attached to the first and three carbon atoms, one hydrogen atom, and one $OH$ group. Since it is attached to two adjacent carbon atoms, the second carbon atom is of $2^{\circ}$.
The third carbon atom is attached to the second carbon atom, one hydrogen atom, and one $OH$ group. Since it is attached to only one adjacent carbon atom, the third carbon atom is of $1^{\circ}$.
From the above observations, it can be concluded that glycerol contains only $1^{\circ}$ and $2^{\circ}$ carbon atoms. Therefore, $3^{\circ}$ carbon atoms are absent in glycerol.
So, the correct answer is Option c.
Note: Glycerol should not be confused with glycol, whose IUPAC name is ethane-$1,2$-diol. The chemical formula for a glycol is $CH_2OH-CH_2OH$.
A $1^{\circ}$ carbon atom is also called a primary carbon atom.
A $2^{\circ}4$ carbon atom is also called a secondary carbon atom.
A $3^{\circ}$ carbon atom is also called a tertiary carbon atom.
Complete step by step solution:
A carbon atom can be of three types - $1^{\circ}, 2^{\circ} , 3^{\circ}$.
$A 1^{\circ}$ carbon is a carbon atom that is not attached to any other carbon atom.
$A 2^{\circ}$ carbon is a carbon atom that is attached to one carbon atom and three other atoms.
$A 3^{\circ}$ carbon is a carbon atom that is attached to two other carbon atoms and another atom.
The structure of propane-1,2,3-triol can be represented as under:

The first carbon atom is attached to the second carbon atom, two hydrogen atoms, and one $OH$ group. Since it is attached to only one carbon atom, the first carbon atom is of $1^{\circ}$.
The second carbon atom is attached to the first and three carbon atoms, one hydrogen atom, and one $OH$ group. Since it is attached to two adjacent carbon atoms, the second carbon atom is of $2^{\circ}$.
The third carbon atom is attached to the second carbon atom, one hydrogen atom, and one $OH$ group. Since it is attached to only one adjacent carbon atom, the third carbon atom is of $1^{\circ}$.
From the above observations, it can be concluded that glycerol contains only $1^{\circ}$ and $2^{\circ}$ carbon atoms. Therefore, $3^{\circ}$ carbon atoms are absent in glycerol.
So, the correct answer is Option c.
Note: Glycerol should not be confused with glycol, whose IUPAC name is ethane-$1,2$-diol. The chemical formula for a glycol is $CH_2OH-CH_2OH$.
A $1^{\circ}$ carbon atom is also called a primary carbon atom.
A $2^{\circ}4$ carbon atom is also called a secondary carbon atom.
A $3^{\circ}$ carbon atom is also called a tertiary carbon atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




