
Glycerol is a:
a.) Monofunctional compound
b.) Difunctional compound
c.) Trifunctional compound
d.) Non – functional compound
Answer
596.1k+ views
Hint: Glycerol has three hydroxyl groups. Functionality of a compound is the presence of functional groups. All the hydroxyl groups are attached to three consecutive carbons.
Complete step by step solution:
Functionality is the presence of functional groups in a molecule. Functionality of a molecule has a decisive influence on its reactivity. A monofunctional molecule possesses one function, a difunctional two, a trifunctional three, etc.
Glycerol is also known as glycerine or glycerin. It is a simple polyol compound. It is a colourless, odourless, viscous liquid and is sweet-tasting and non-toxic. The backbone of glycerol is found in lipids known as glycerides. It is also used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Due to the presence of three hydroxyl groups, glycerol is miscible in water and hygroscopic in nature. Glycerol is an achiral compound.
The molecular formula of glycerol is \[{C_3}{H_5}{(OH)_3}\]. The structure of glycerol is:
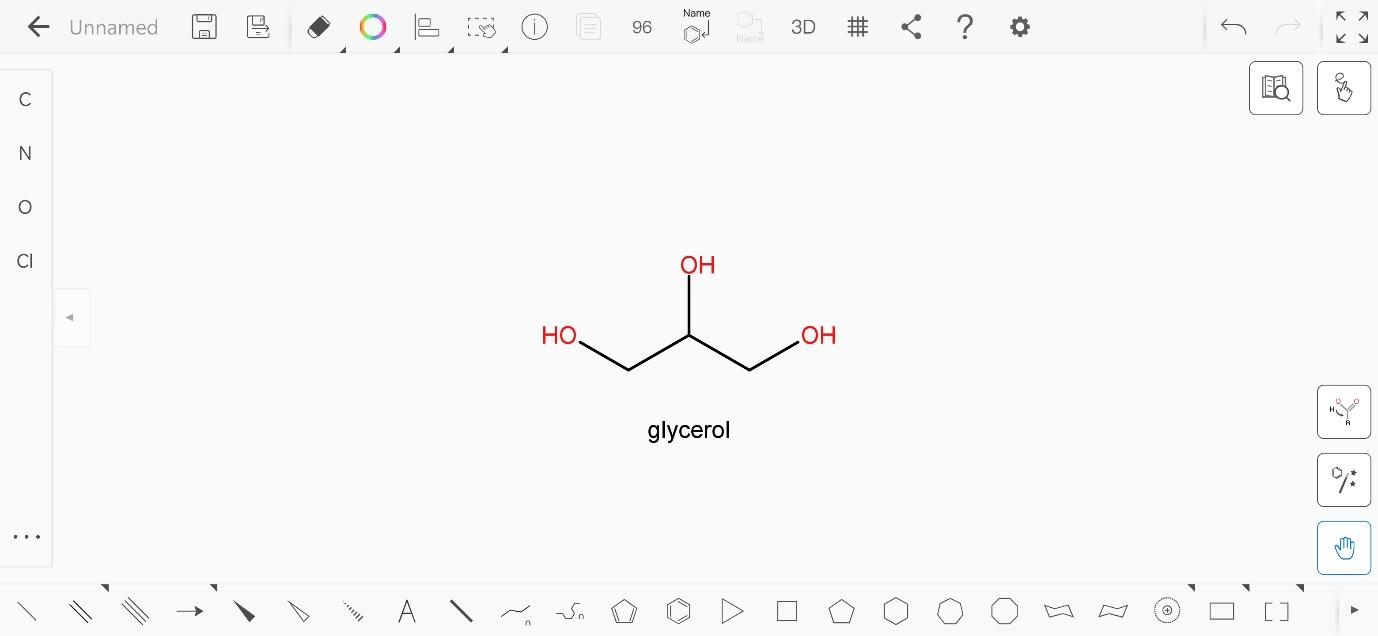
It has three carbons, each of which has one hydroxyl group attached to them. Hence, it has a total of three hydroxyl groups. Thus, glycerol is a trifunctional compound.
Hence, the correct answer is (C).
Note: Remember that the total number of functional groups are counted for the functionality of a compound, not the total distinct functional groups.
Complete step by step solution:
Functionality is the presence of functional groups in a molecule. Functionality of a molecule has a decisive influence on its reactivity. A monofunctional molecule possesses one function, a difunctional two, a trifunctional three, etc.
Glycerol is also known as glycerine or glycerin. It is a simple polyol compound. It is a colourless, odourless, viscous liquid and is sweet-tasting and non-toxic. The backbone of glycerol is found in lipids known as glycerides. It is also used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Due to the presence of three hydroxyl groups, glycerol is miscible in water and hygroscopic in nature. Glycerol is an achiral compound.
The molecular formula of glycerol is \[{C_3}{H_5}{(OH)_3}\]. The structure of glycerol is:

It has three carbons, each of which has one hydroxyl group attached to them. Hence, it has a total of three hydroxyl groups. Thus, glycerol is a trifunctional compound.
Hence, the correct answer is (C).
Note: Remember that the total number of functional groups are counted for the functionality of a compound, not the total distinct functional groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




