
\[Glycerol\xrightarrow{KHS{{O}_{4}}}A\xrightarrow{HOCl}B\]
A and B are respectively
(a)
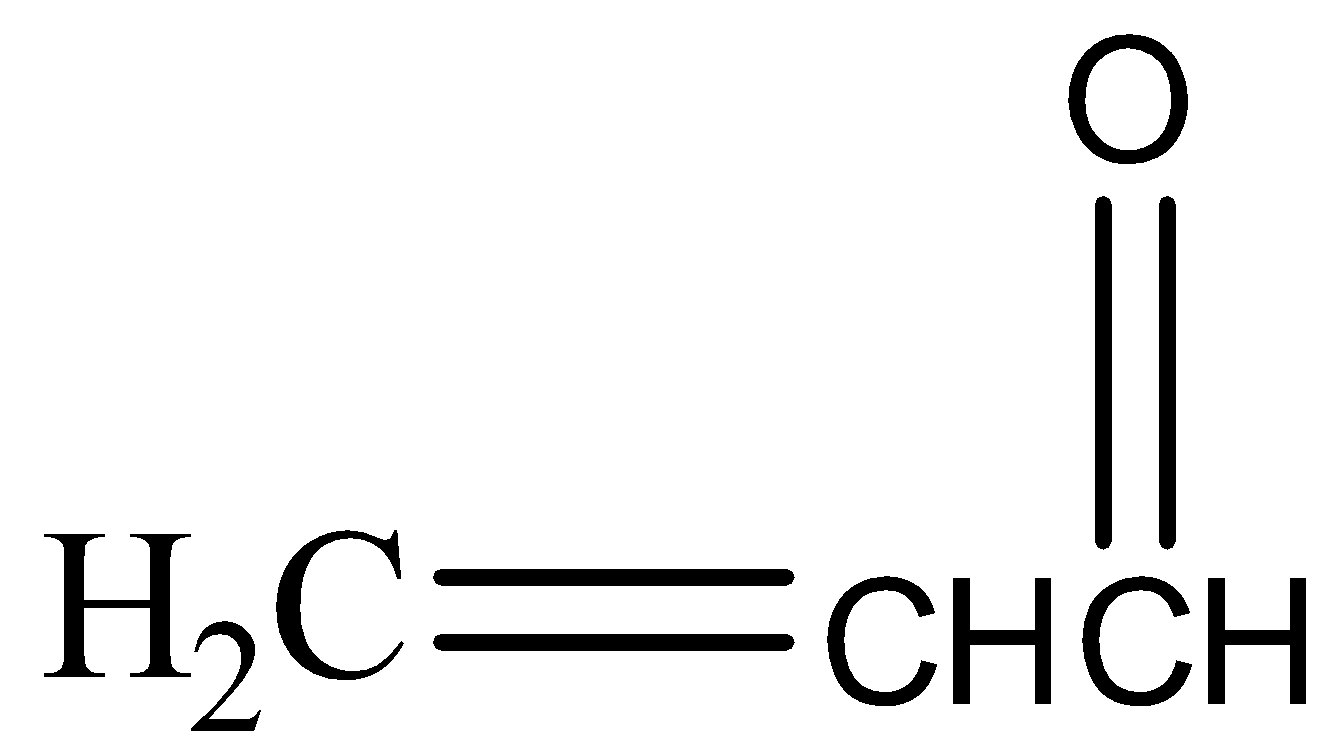 ,
,
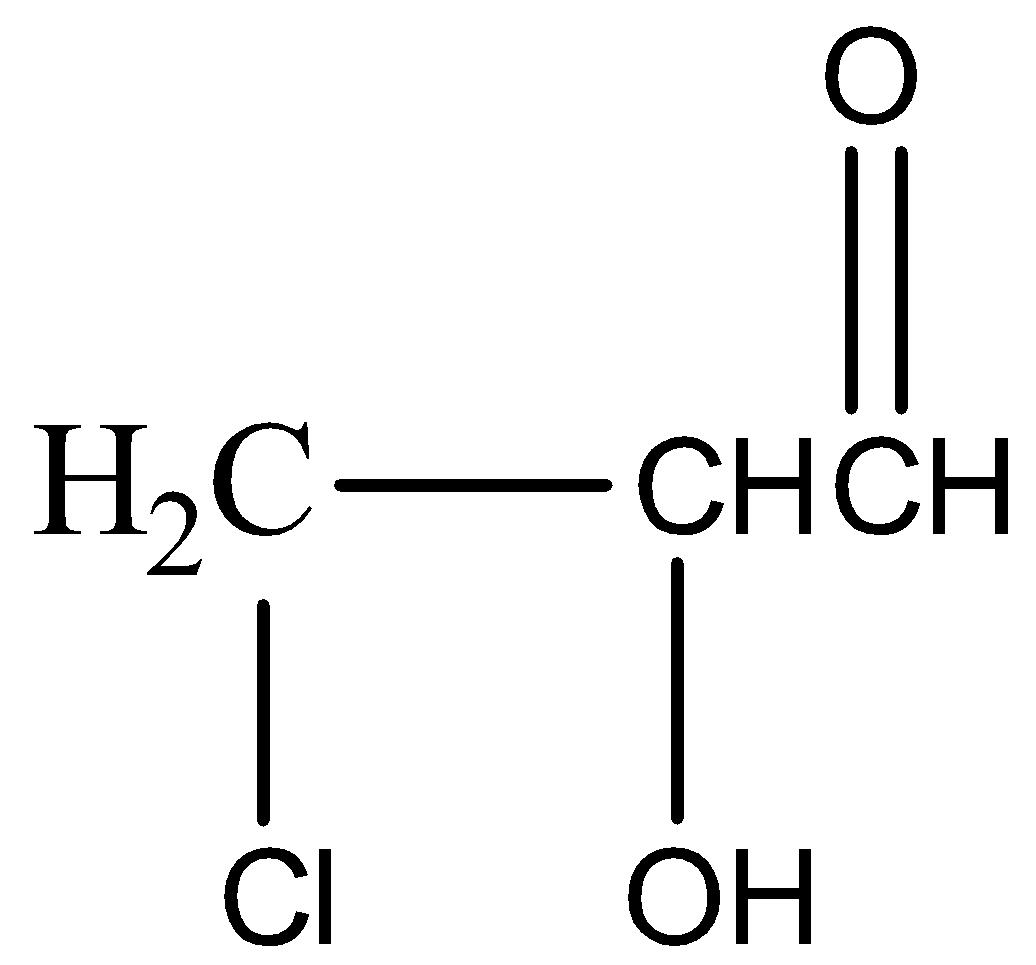
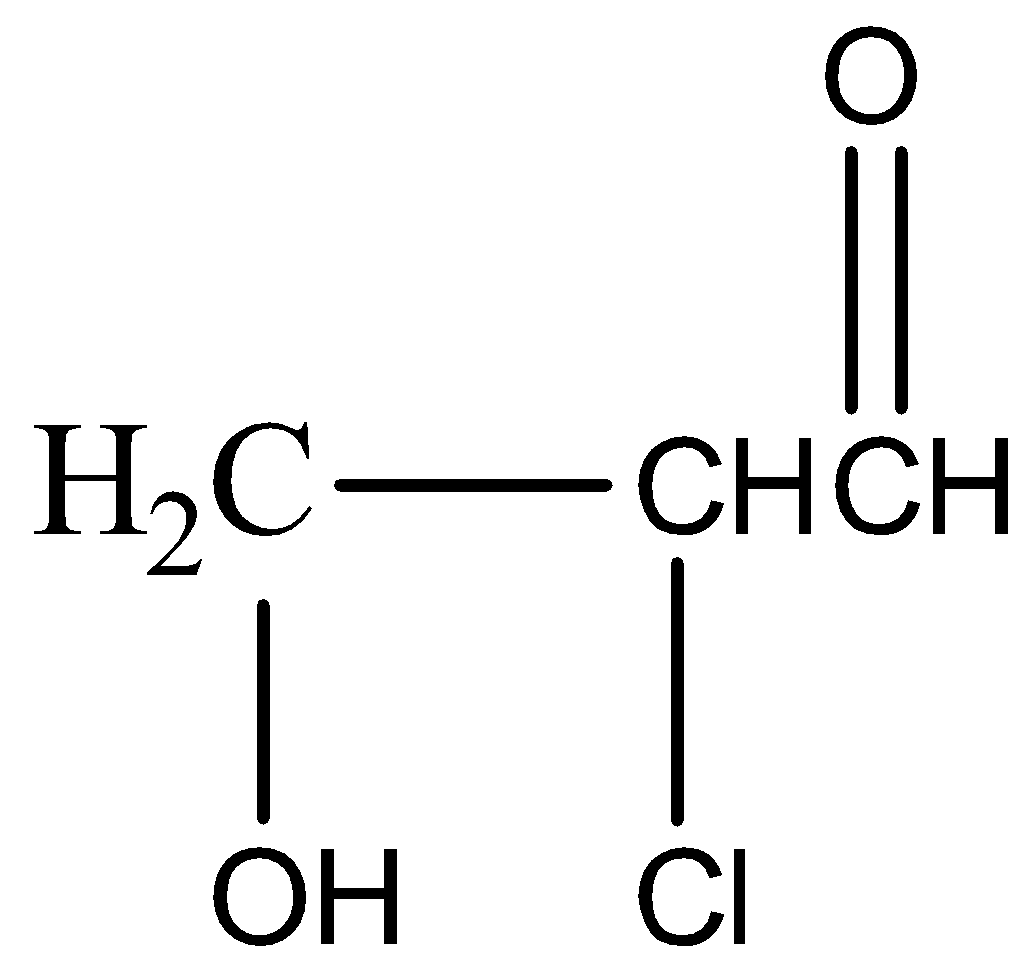
(b)
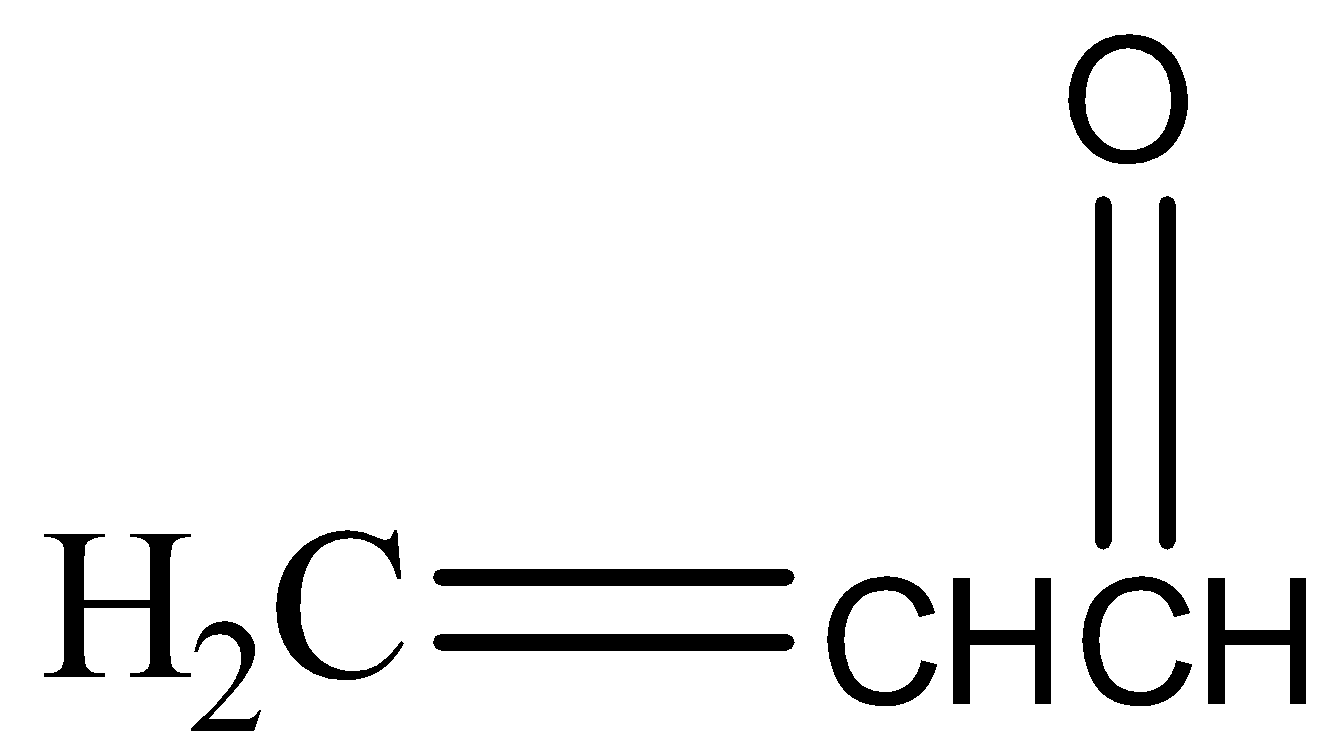 ,
,
(c)\[C{{H}_{3}}C{{H}_{2}}CHO\],
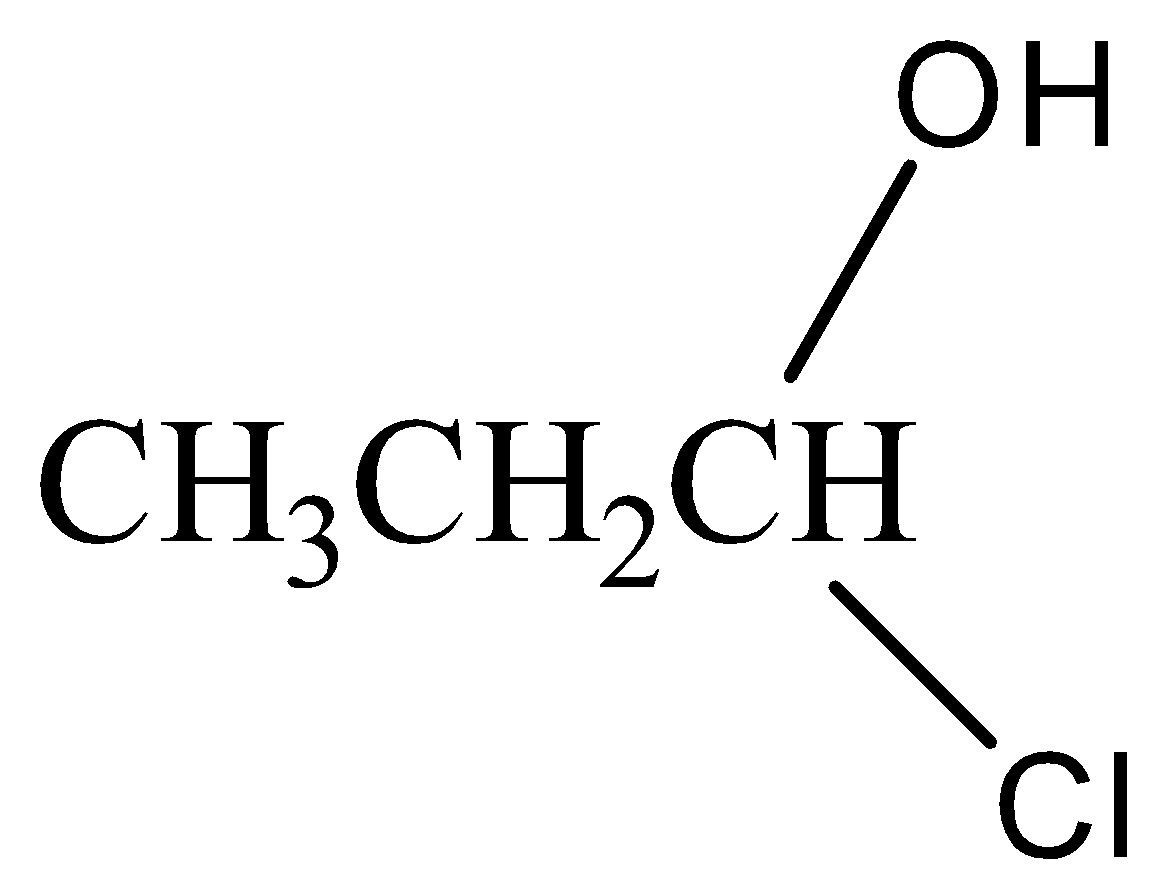
(d)
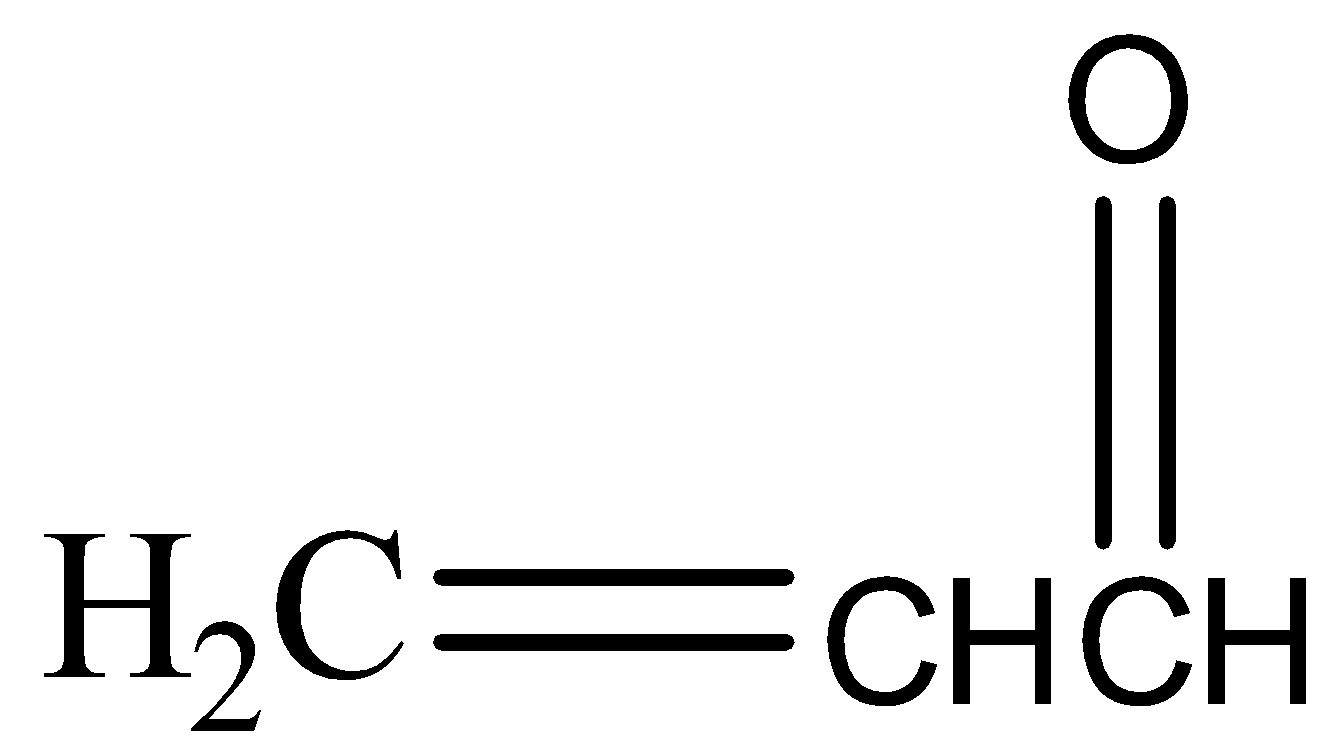 ,
,
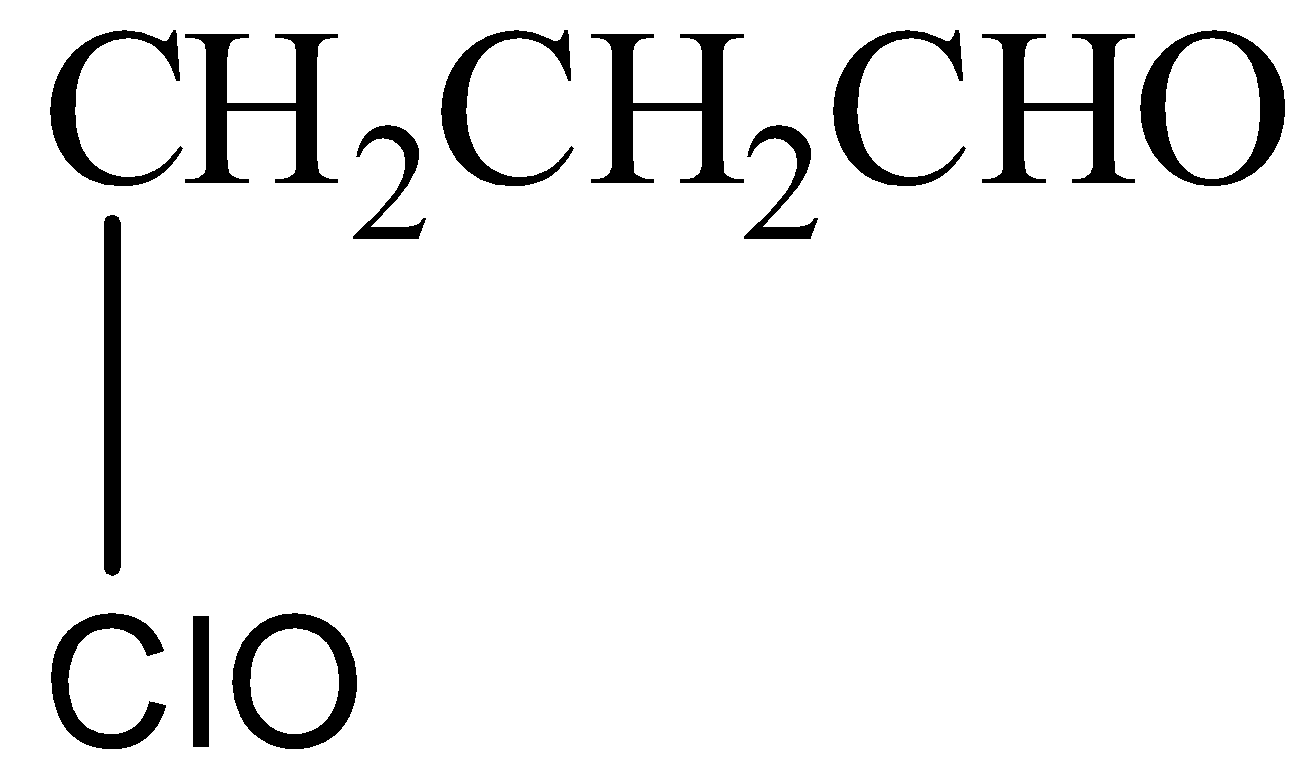
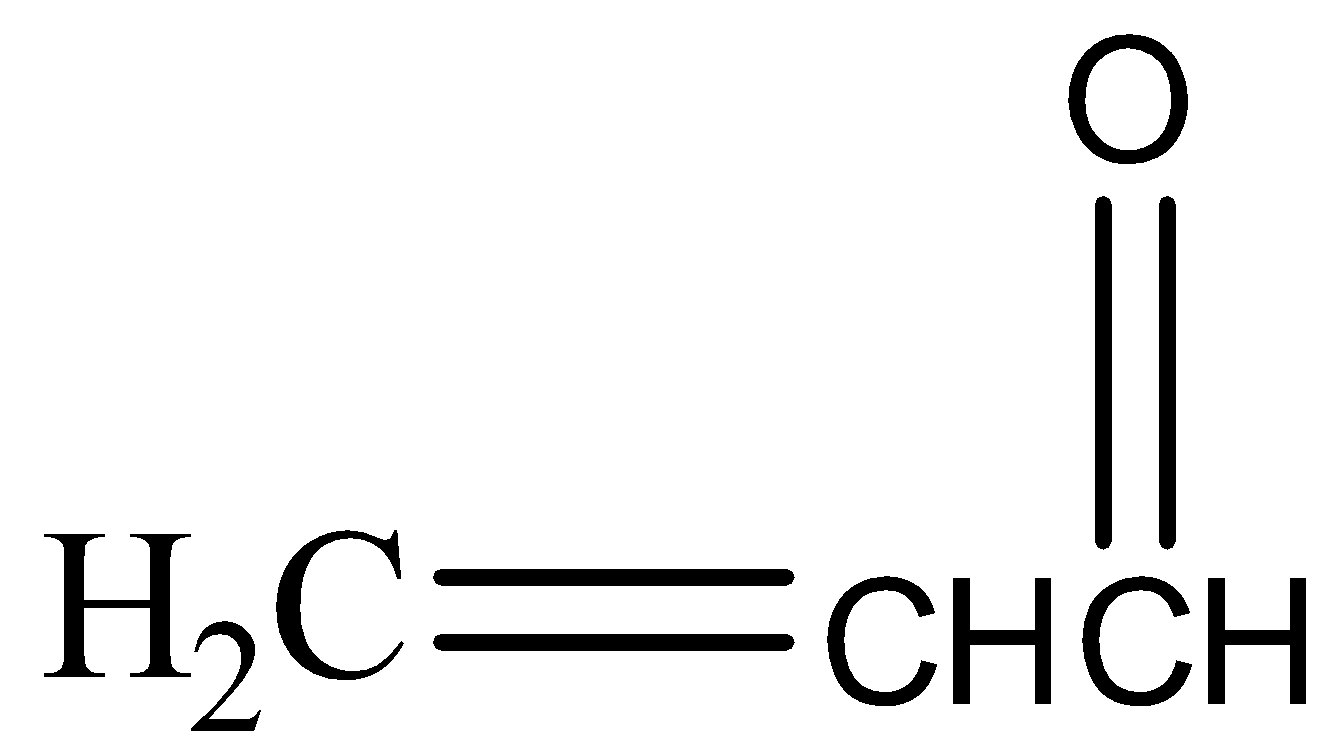

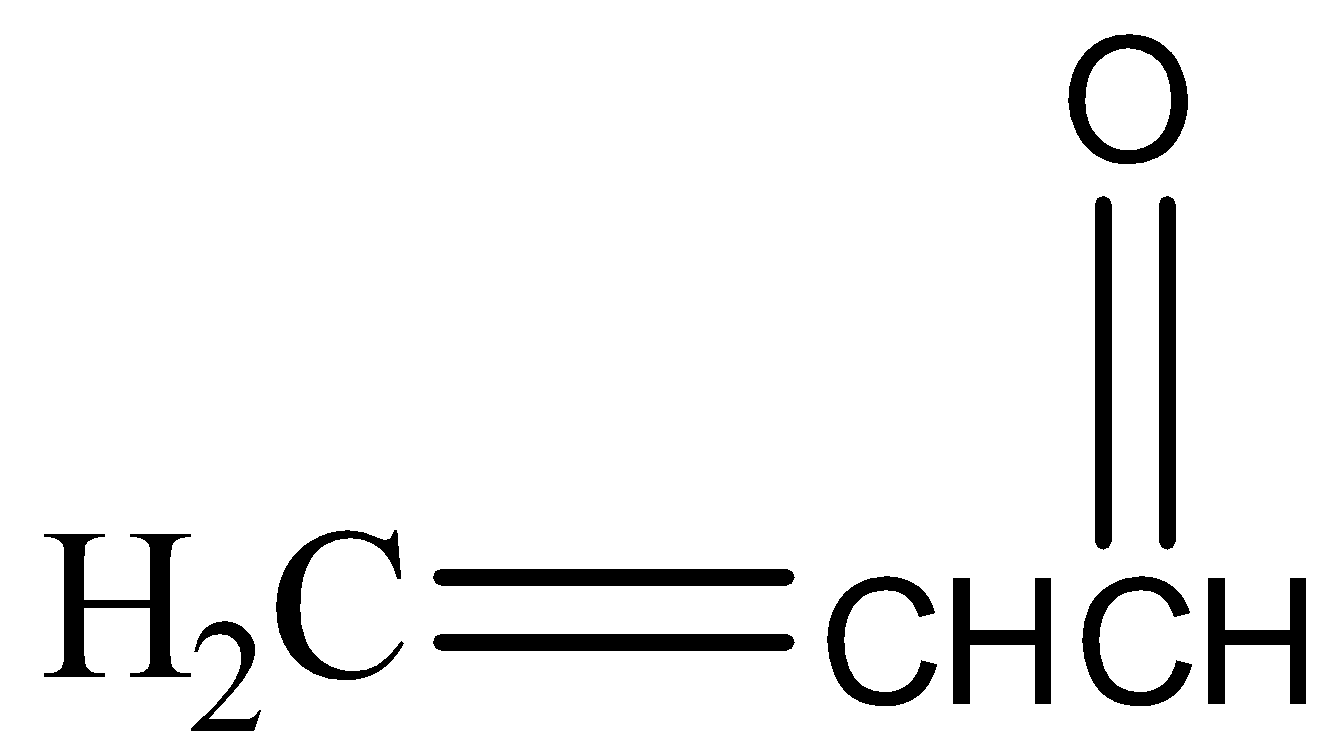


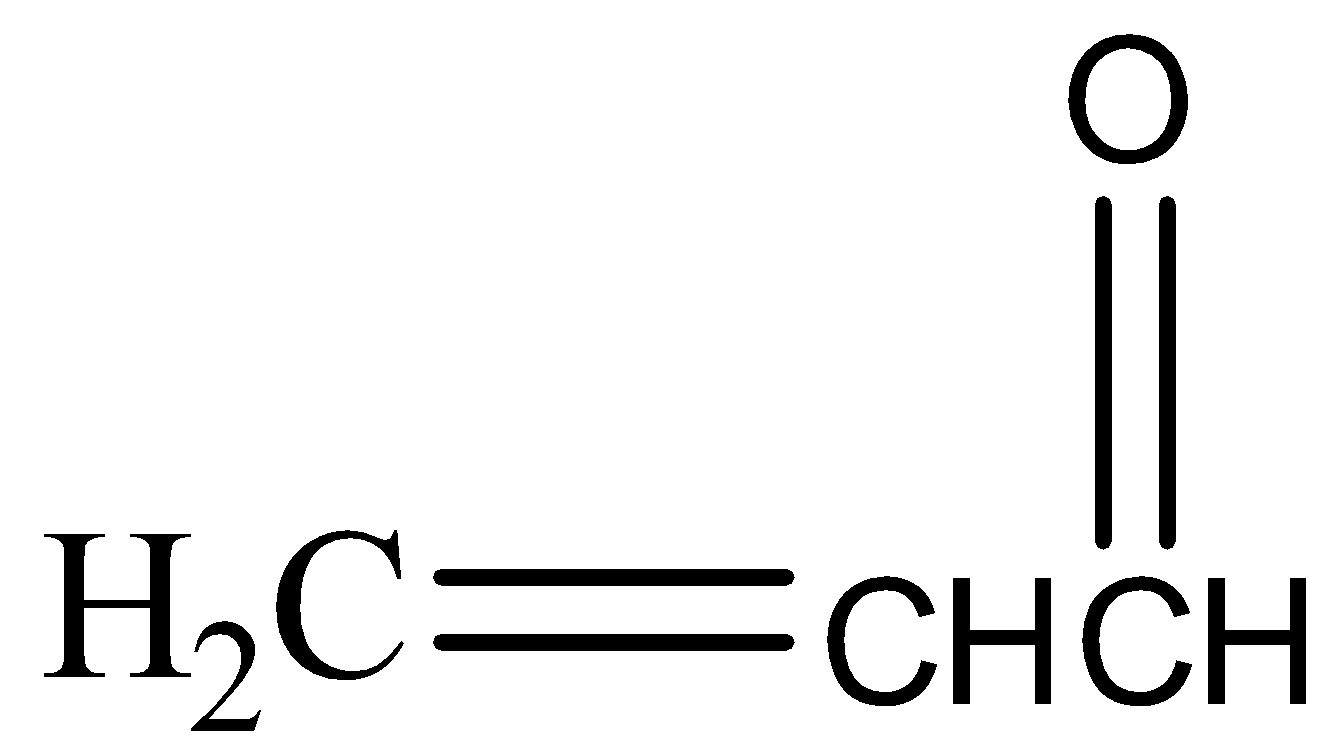
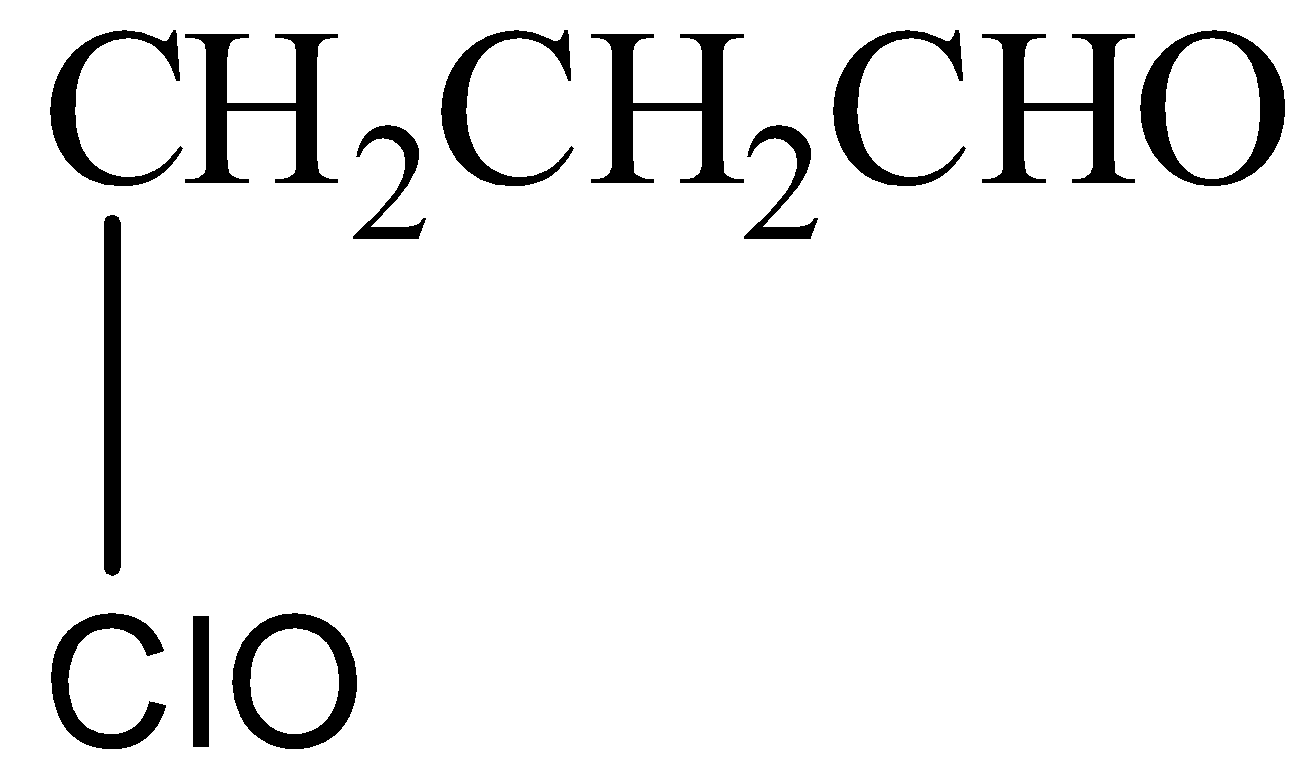
Answer
594.9k+ views
Hint: Glycerol has 3 alkyl groups each attached with an OH group to it. The hydroxyl groups can be easily removed to form a tautomerism product.
Complete step by step solution:
\[KHS{{O}_{4}}\] has a dehydrating property. It abstracts water molecules from glycerol and also oxidises it to unsaturated aldehyde or acrylic aldehyde or acrolein( a tautomerism product). In the presence of HOCl the double bond shifts to the middle carbon and forms a carbanion and a carbocation. \[O{{H}^{-}}\]of HOCl forms a bond with the carbocation and \[C{{l}^{+}}\] attacks the carbanion giving us the final product.
The mechanism of the reaction is shown as
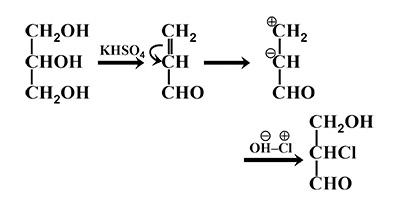
So, the correct answer is “Option B”.
Additional Information:
-Carbocation is a species in which the carbon atom has a positive charge on it and three bonds. These are basically carbon cations. These are very reactive species and are unstable. Their instability is due to the incomplete octet. Carbanions are carbon with negative charge. It is also trivalent. Like carbocation these are also highly unstable.
-Acrolein formed is a dehydrated product of glycerol when it is reacted with \[KHS{{O}_{4}}\]. It is a colourless liquid with an acrid smell. This actually formed by the tautomerization of glycerol. Tautomers are structural isomers in which the chemical compound can readily convert. Tautomerism is mainly shown by nucleic acids and amino acids. There are chances that it is confused with resonance structures. But both are different. Tautomers can be distinguished by spectral data. Resonance structures cannot be distinguished by spectral data. One of the examples is keto-enol tautomerism. Another name for tautomerism is thigmotropism.
Note: Water molecule is abstracted by \[KHS{{O}_{4}}\] to give acrylic aldehyde. This then reacts with HOCl to give a substituted product. Which should note that the double bond of the unsaturated aldehyde is shifted to the middle carbon and the \[C{{l}^{+}}\] attacks this carbanion. \[O{{H}^{-}}\] attacks the carbocation formed.
Complete step by step solution:
\[KHS{{O}_{4}}\] has a dehydrating property. It abstracts water molecules from glycerol and also oxidises it to unsaturated aldehyde or acrylic aldehyde or acrolein( a tautomerism product). In the presence of HOCl the double bond shifts to the middle carbon and forms a carbanion and a carbocation. \[O{{H}^{-}}\]of HOCl forms a bond with the carbocation and \[C{{l}^{+}}\] attacks the carbanion giving us the final product.
The mechanism of the reaction is shown as

So, the correct answer is “Option B”.
Additional Information:
-Carbocation is a species in which the carbon atom has a positive charge on it and three bonds. These are basically carbon cations. These are very reactive species and are unstable. Their instability is due to the incomplete octet. Carbanions are carbon with negative charge. It is also trivalent. Like carbocation these are also highly unstable.
-Acrolein formed is a dehydrated product of glycerol when it is reacted with \[KHS{{O}_{4}}\]. It is a colourless liquid with an acrid smell. This actually formed by the tautomerization of glycerol. Tautomers are structural isomers in which the chemical compound can readily convert. Tautomerism is mainly shown by nucleic acids and amino acids. There are chances that it is confused with resonance structures. But both are different. Tautomers can be distinguished by spectral data. Resonance structures cannot be distinguished by spectral data. One of the examples is keto-enol tautomerism. Another name for tautomerism is thigmotropism.
Note: Water molecule is abstracted by \[KHS{{O}_{4}}\] to give acrylic aldehyde. This then reacts with HOCl to give a substituted product. Which should note that the double bond of the unsaturated aldehyde is shifted to the middle carbon and the \[C{{l}^{+}}\] attacks this carbanion. \[O{{H}^{-}}\] attacks the carbocation formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




