
Gold leaf electroscope is used:
A.) to measure the electric charge
B.) to detect and test small electric charges
C.) to produce electric current
D.) to produce electric charges
Answer
602.1k+ views
Hint: The gold-leaf electroscope is used to detect, measure and find the nature of a charge. This device works on the principle repulsion experienced between like charges.
Complete step by step answer:
A gold-leaf type electroscope is a device used to detect the presence of electric charge on a body and its relative amount. The electroscope is usually constructed with a metal plate or sphere at the top of a metal post (electrode) with thin foil leaves (e.g., gold) hanging from the bottom of the post. When the electrode is charged by induction or by contact, the leaves acquire similar electric charges and repel each other due to the Coulomb force.
Their separation is a direct indication of the net charge stored on them. To protect the gold leaves from drafts of air they are typically enclosed in a glass bottle or glass-walled chamber, usually open at the bottom and mounted over a conductive base. Often there are grounded metal plates or foil strips in the bottle flanking the gold leaves on either side. These are safety measures.
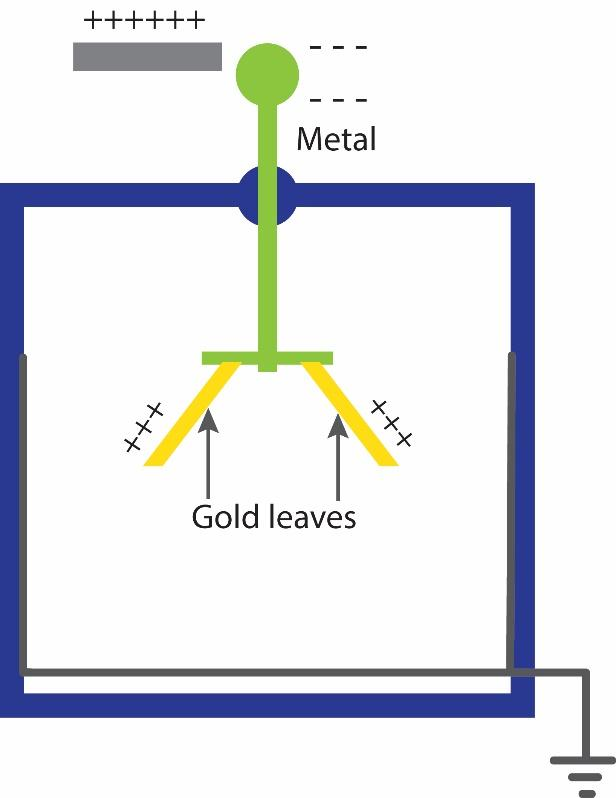
If an excessive charge is applied to the delicate gold leaves, they will touch the grounding plates and discharge before tearing. They also capture charge leaking through the air that could accumulate on the glass walls, and increase the sensitivity of the instrument.
Since electroscopes are used to detect the presence of charge. So, through it we can find whether a body is charged or uncharged. The degree of divergence is an indicator of the amount of charge i.e., more the charge, more will be the divergence.
The applications of gold leaf electroscope are:
1. To detect charges.
2. To identify the nature of charges.
3. Identify a body as conductor or insulator.
So, the correct option is B i.e., to detect and test small electric charges.
Note: Students must take care of the fact that a gold leaf electroscope is used to detect only the presence of charges, not the quantity of the charge on the charged rod. We can find that the body is charged or uncharged.
Complete step by step answer:
A gold-leaf type electroscope is a device used to detect the presence of electric charge on a body and its relative amount. The electroscope is usually constructed with a metal plate or sphere at the top of a metal post (electrode) with thin foil leaves (e.g., gold) hanging from the bottom of the post. When the electrode is charged by induction or by contact, the leaves acquire similar electric charges and repel each other due to the Coulomb force.
Their separation is a direct indication of the net charge stored on them. To protect the gold leaves from drafts of air they are typically enclosed in a glass bottle or glass-walled chamber, usually open at the bottom and mounted over a conductive base. Often there are grounded metal plates or foil strips in the bottle flanking the gold leaves on either side. These are safety measures.

If an excessive charge is applied to the delicate gold leaves, they will touch the grounding plates and discharge before tearing. They also capture charge leaking through the air that could accumulate on the glass walls, and increase the sensitivity of the instrument.
Since electroscopes are used to detect the presence of charge. So, through it we can find whether a body is charged or uncharged. The degree of divergence is an indicator of the amount of charge i.e., more the charge, more will be the divergence.
The applications of gold leaf electroscope are:
1. To detect charges.
2. To identify the nature of charges.
3. Identify a body as conductor or insulator.
So, the correct option is B i.e., to detect and test small electric charges.
Note: Students must take care of the fact that a gold leaf electroscope is used to detect only the presence of charges, not the quantity of the charge on the charged rod. We can find that the body is charged or uncharged.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




