
Graphite is used in pencils. It leaves a black trace of writing on paper because it consists of layers of carbon atoms that:
A.Are wound inside, like a long thread
B.Are attracted by paper material
C.Fall easily
D.Slide over each other
Answer
566.7k+ views
Hint: We have to know that a crystalline form of the carbon with the atoms of carbon arranged in a hexagonal structure is graphite. The colour of graphite is greyish black and is an opaque substance. Graphite is made up of carbon. A huge amount of pressure has to be applied to break the covalent bonds in graphite.
Complete answer:
We must remember that the graphite contains carbon triple bonded molecular lattices, which are attached through covalent bonds. The atoms of carbon create layers with hexagonal arrangement of atoms. The different layers of carbon atoms present in graphite are bounded by weak van der Waals forces. In graphite, an atom of carbon is $s{p^2}$ hybridized and each carbon is bonded to three other carbon atoms by leading to hexagonal rings. Each carbon atom has an unhybridized p-orbital that undergoes sideways overlap to produce three $p\pi - p\pi $ double bonds. Hence, graphite has a two-dimensional sheet-like structure consisting of a number of hexagonal rings fused together. The sheet-like structure gives graphite a soft material.
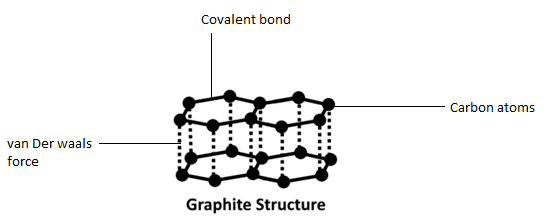
From the above explanation, we saw that graphite contains layered structures of layers which are linked by van der Waal forces. The cleaning between the layers is done by graphite, thus, it is so slippery and soft. This is the reason why graphite is used in pencil and as lubricants in machines which operate at high temperature.
Therefore, the option (D) is correct.
Note:
We need to know that a crystalline form of the carbon with the atoms of arrangement of carbons in a hexagonal structure is graphite. The colour of graphite is greyish black and graphite is an opaque substance. Graphite is made up of carbon. We could utilize graphite in electrodes, dry cells. We could utilize Graphite functions as anode in the electrolysis of alumina. Graphite avoids the liberation of a gas in the electrometallurgy of aluminum.
Complete answer:
We must remember that the graphite contains carbon triple bonded molecular lattices, which are attached through covalent bonds. The atoms of carbon create layers with hexagonal arrangement of atoms. The different layers of carbon atoms present in graphite are bounded by weak van der Waals forces. In graphite, an atom of carbon is $s{p^2}$ hybridized and each carbon is bonded to three other carbon atoms by leading to hexagonal rings. Each carbon atom has an unhybridized p-orbital that undergoes sideways overlap to produce three $p\pi - p\pi $ double bonds. Hence, graphite has a two-dimensional sheet-like structure consisting of a number of hexagonal rings fused together. The sheet-like structure gives graphite a soft material.

From the above explanation, we saw that graphite contains layered structures of layers which are linked by van der Waal forces. The cleaning between the layers is done by graphite, thus, it is so slippery and soft. This is the reason why graphite is used in pencil and as lubricants in machines which operate at high temperature.
Therefore, the option (D) is correct.
Note:
We need to know that a crystalline form of the carbon with the atoms of arrangement of carbons in a hexagonal structure is graphite. The colour of graphite is greyish black and graphite is an opaque substance. Graphite is made up of carbon. We could utilize graphite in electrodes, dry cells. We could utilize Graphite functions as anode in the electrolysis of alumina. Graphite avoids the liberation of a gas in the electrometallurgy of aluminum.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




