
What is the ground-state term symbol for the aluminium atom in a magnetic field?
Answer
489.9k+ views
Hint: The term symbol in quantum mechanics is an abbreviated description of the angular momentum quantum numbers in a multi-electron system. Every energy level is not only described by its configuration but also its term symbol. The term symbol usually assumes LS coupling.
Complete Step By Step Answer:
The term symbol has a form of: $ ^{2S + 1}{L_J} $
Where $ 2S + 1 $ is the spin multiplicity, L is the orbital quantum number having values S, P, D, F, G, etc. and J is the total angular momentum quantum number. The value of J ranges from $ {J_{\max }} - {J_{\min }} $ (max to min) . The value of $ {J_{\max }} = |L + S| $ and $ {J_{\min }} = |L - S| $
The spin multiplicity or the total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $ for I no. of electrons. And total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $ for I no. of electrons. If the value of L =0,1,2,3,4, etc. it corresponds to L = S,P,D,F,G, etc, respectively.
We are given the atom Aluminum. The electronic configuration of Aluminum is given as: $ Al:[Ne]3{s^2}3{p^1} $
Diagrammatically it is given as:
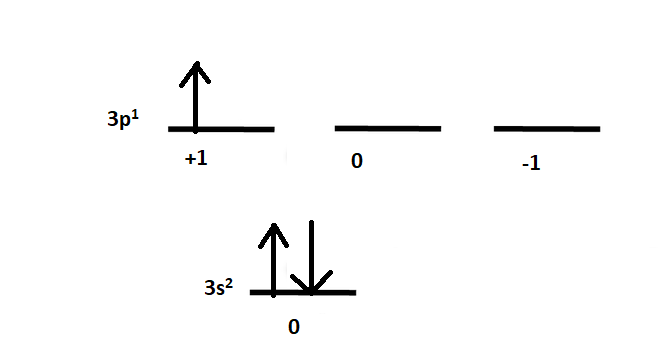
The 3s orbital has two paired electrons and 3p has one unpaired electron. Let us find the term symbols for each orbital one by one.
TERM SYMBOL FOR 3S ORBITAL:
Now, since we know the electronic configuration let us find the term symbols.
The total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $
For the given configuration of electrons the value of $ S = \dfrac{1}{2} - \dfrac{1}{2} = 0 $
The spin multiplicity will be equal to $ {S_m} = 2S + 1 = 2(0) + 1 = 1 $ . Spin multiplicity = 1 indicates Singlet state.
The value of total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $
The doubly occupied 3s orbital will have a $ {m_l} = 0 $ . The total angular momentum quantum number L will be: $ L = |0| = 0 \to S $
The term symbol until now can be written as $ ^1S $
The value of J will be from $ {J_{\max }} = |L + S| $ to $ {J_{\min }} = |L - S| $ i.e. from $ {J_{\min }} = |0 - 0| = 0 $ to $ {J_{\max }} = |0 - 0| = 0 $ . Therefore, the value of J will be $ J = 0 $ . The term symbol for 3s orbital will be $ ^1{S_0} $
TERM SYMBOL FOR 3p ORBITAL:
Now, since we know the electronic configuration let us find the term symbols.
The total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $
For the given configuration of electrons the value of $ S = \dfrac{1}{2} = \dfrac{1}{2} $
The spin multiplicity will be equal to $ {S_m} = 2S + 1 = 2\left( {\dfrac{1}{2}} \right) + 1 = 2 $ . Spin multiplicity = 2 indicates Doublet state.
The value of total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $
The singly occupied orbital will have a $ {m_l} = + 1 $ . The total angular momentum quantum number L will be: $ L = | + 1| = 1 \to P $
The term symbol until now can be written as $ ^2P $
The value of J will be from $ {J_{\max }} = |L + S| $ to $ {J_{\min }} = |L - S| $ i.e. from $ {J_{\min }} = |1 - \dfrac{1}{2}| = \dfrac{1}{2} $ to $ {J_{\max }} = |1 + \dfrac{1}{2}| = \dfrac{3}{2} $ . Therefore, the value of J will be $ J = \dfrac{1}{2},\dfrac{3}{2} $
The term symbols for 3p orbitals will thus will have two values: $ ^2{P_{\dfrac{1}{2}}}{,^2}{P_{\dfrac{3}{2}}} $
We are asked to find the Ground state term symbol, according to Hund’s rule:
- The term with the largest S is more stable, unless all have the same value of S.
- For terms having the same value of S and L, the subshell that has less than half filled electrons will have the smallest J and vice versa. If it has exactly half-filled electrons J will be 0.
In the given configuration the values of S and L are same, and 3p is less than half filled orbital, therefore 1/2 is more stable than 3/2. The final ground state term symbol is $ ^2{P_{1/2}} $ . This is the required answer.
Note:
If we are asked the ground state term symbol, the value of J will be $ {J_{\min }} = |L - S| $ for less than half filled orbitals and $ {J_{\max }} = |L + S| $ for more than half filled orbitals. In this case the orbital is less than half filled, hence the value of J will be $ {J_{\min }} = |5 - 1| = 4 $ and the ground state term symbol will be $ ^3{H_4} $
Complete Step By Step Answer:
The term symbol has a form of: $ ^{2S + 1}{L_J} $
Where $ 2S + 1 $ is the spin multiplicity, L is the orbital quantum number having values S, P, D, F, G, etc. and J is the total angular momentum quantum number. The value of J ranges from $ {J_{\max }} - {J_{\min }} $ (max to min) . The value of $ {J_{\max }} = |L + S| $ and $ {J_{\min }} = |L - S| $
The spin multiplicity or the total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $ for I no. of electrons. And total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $ for I no. of electrons. If the value of L =0,1,2,3,4, etc. it corresponds to L = S,P,D,F,G, etc, respectively.
We are given the atom Aluminum. The electronic configuration of Aluminum is given as: $ Al:[Ne]3{s^2}3{p^1} $
Diagrammatically it is given as:

The 3s orbital has two paired electrons and 3p has one unpaired electron. Let us find the term symbols for each orbital one by one.
TERM SYMBOL FOR 3S ORBITAL:
Now, since we know the electronic configuration let us find the term symbols.
The total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $
For the given configuration of electrons the value of $ S = \dfrac{1}{2} - \dfrac{1}{2} = 0 $
The spin multiplicity will be equal to $ {S_m} = 2S + 1 = 2(0) + 1 = 1 $ . Spin multiplicity = 1 indicates Singlet state.
The value of total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $
The doubly occupied 3s orbital will have a $ {m_l} = 0 $ . The total angular momentum quantum number L will be: $ L = |0| = 0 \to S $
The term symbol until now can be written as $ ^1S $
The value of J will be from $ {J_{\max }} = |L + S| $ to $ {J_{\min }} = |L - S| $ i.e. from $ {J_{\min }} = |0 - 0| = 0 $ to $ {J_{\max }} = |0 - 0| = 0 $ . Therefore, the value of J will be $ J = 0 $ . The term symbol for 3s orbital will be $ ^1{S_0} $
TERM SYMBOL FOR 3p ORBITAL:
Now, since we know the electronic configuration let us find the term symbols.
The total spin angular momentum can be given as: $ S = |{M_S}| = |\sum\limits_i {{m_{s,i}}} | $
For the given configuration of electrons the value of $ S = \dfrac{1}{2} = \dfrac{1}{2} $
The spin multiplicity will be equal to $ {S_m} = 2S + 1 = 2\left( {\dfrac{1}{2}} \right) + 1 = 2 $ . Spin multiplicity = 2 indicates Doublet state.
The value of total orbital angular momentum quantum number L can be given as: $ L = |{M_L}| = |\sum\limits_i {{m_{l,i}}} | $
The singly occupied orbital will have a $ {m_l} = + 1 $ . The total angular momentum quantum number L will be: $ L = | + 1| = 1 \to P $
The term symbol until now can be written as $ ^2P $
The value of J will be from $ {J_{\max }} = |L + S| $ to $ {J_{\min }} = |L - S| $ i.e. from $ {J_{\min }} = |1 - \dfrac{1}{2}| = \dfrac{1}{2} $ to $ {J_{\max }} = |1 + \dfrac{1}{2}| = \dfrac{3}{2} $ . Therefore, the value of J will be $ J = \dfrac{1}{2},\dfrac{3}{2} $
The term symbols for 3p orbitals will thus will have two values: $ ^2{P_{\dfrac{1}{2}}}{,^2}{P_{\dfrac{3}{2}}} $
We are asked to find the Ground state term symbol, according to Hund’s rule:
- The term with the largest S is more stable, unless all have the same value of S.
- For terms having the same value of S and L, the subshell that has less than half filled electrons will have the smallest J and vice versa. If it has exactly half-filled electrons J will be 0.
In the given configuration the values of S and L are same, and 3p is less than half filled orbital, therefore 1/2 is more stable than 3/2. The final ground state term symbol is $ ^2{P_{1/2}} $ . This is the required answer.
Note:
If we are asked the ground state term symbol, the value of J will be $ {J_{\min }} = |L - S| $ for less than half filled orbitals and $ {J_{\max }} = |L + S| $ for more than half filled orbitals. In this case the orbital is less than half filled, hence the value of J will be $ {J_{\min }} = |5 - 1| = 4 $ and the ground state term symbol will be $ ^3{H_4} $
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




