
${{H}_{3}}P{{O}_{2}}$ is the molecular formula of an acid of phosphorus. Its name and basicity respectively are:
(A) Metaphosphorous acid and one
(B) Hypophosphorous acid and one
(C) Metaphosphoric acid and one
(D) Hypophosphoric acid and one
Answer
588k+ views
Hint: The acids containing the element oxygen are oxoacids. These oxoacids contain at least one other element and at least one hydrogen atom bonded to oxygen. Like phosphorus, sulfur and halogens will form these types of oxoacids. Sometimes these oxoacids are also called oxyacids.
Complete step by step solution:
Phosphorus forms many numbers of oxoacids. For example, ${{H}_{3}}P{{O}_{2}},{{H}_{3}}P{{O}_{3}},{{H}_{3}}P{{O}_{4}}$ , etc. In oxoacids of phosphorus, is a tetrahedral surrounded by other atoms. All these oxoacids of phosphorus are known to form at least one P-O bond and one P-OH bond.
P-P bonds or P-H bonds are also found including the P-O bonds and P-OH bonds in oxoacids of phosphorus. In these oxoacids, the phosphorus oxidation number is less than +5, also exhibits a lower and higher oxidation state.
the oxidation of P in ${{H}_{3}}P{{O}_{2}}$is +1. So, hypo- indicates the lower oxidation state of phosphorus. Hence, the name of the compound is Hypophosphorous.
This Hypophosphorous contains one P-OH bond from the diagram observed that the basicity of the compound is one(1).
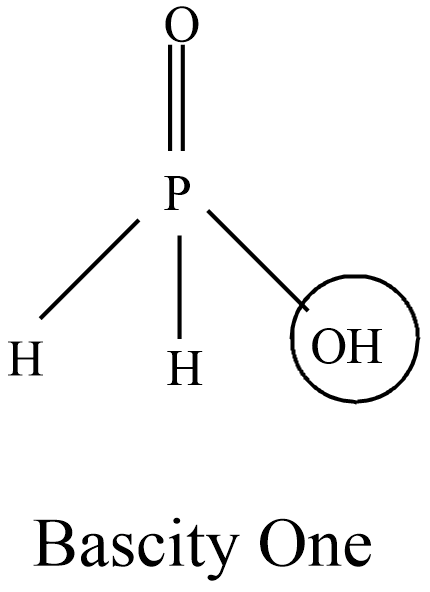
Note: The oxyacids of phosphorus which contain P-H bonds have strong reducing properties. Out of all of the oxoacids of phosphorus, hypophosphorous acid is a good reducing agent which contains two P-H bonds and reduces. The P-H bonds are not ionizable to give ${{H}^{+}}$ do not play a role in basicity.
Complete step by step solution:
Phosphorus forms many numbers of oxoacids. For example, ${{H}_{3}}P{{O}_{2}},{{H}_{3}}P{{O}_{3}},{{H}_{3}}P{{O}_{4}}$ , etc. In oxoacids of phosphorus, is a tetrahedral surrounded by other atoms. All these oxoacids of phosphorus are known to form at least one P-O bond and one P-OH bond.
P-P bonds or P-H bonds are also found including the P-O bonds and P-OH bonds in oxoacids of phosphorus. In these oxoacids, the phosphorus oxidation number is less than +5, also exhibits a lower and higher oxidation state.
| Name | Formula | The oxidation state of phosphorus | Characteristics bonds and their number |
| Hypophosphorous | ${{H}_{3}}P{{O}_{2}}$ | +1 | One P-OHTwo P-HOne P=O |
| Orthophosphorous | \[{{H}_{3}}P{{O}_{3}}\] | +3 | Two P-OHOne P-HOne P=O |
| Pyrophosphorous | ${{H}_{4}}{{P}_{2}}{{O}_{5}}$ | +3 | Two P-OHTwo P-HTwo P=O |
| Orthophosphoric acid | ${{H}_{3}}P{{O}_{4}}$ | +5 | Three P-OHOne P=O |
| Metaphosphoric | ${{(HP{{O}_{3}})}_{n}}$ | +5 | Three P-OHThree P=OThree P-O-P |
the oxidation of P in ${{H}_{3}}P{{O}_{2}}$is +1. So, hypo- indicates the lower oxidation state of phosphorus. Hence, the name of the compound is Hypophosphorous.
This Hypophosphorous contains one P-OH bond from the diagram observed that the basicity of the compound is one(1).

Note: The oxyacids of phosphorus which contain P-H bonds have strong reducing properties. Out of all of the oxoacids of phosphorus, hypophosphorous acid is a good reducing agent which contains two P-H bonds and reduces. The P-H bonds are not ionizable to give ${{H}^{+}}$ do not play a role in basicity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




