
What happens when benzene reacts with $ C{H_3}Cl $ ?
Answer
507.9k+ views
Hint :Alkylation and acylation processes are the two most common Friedel-Crafts reactions. In the year $ 1877 $ , French chemist Charles Friedel and American chemist James Crafts developed these reactions.
Complete Step By Step Answer:
Toluene is formed when benzene interacts with chloromethane ( $ C{H_3}Cl $ ) in the presence of anhydrous aluminium chloride ( $ AlC{l_3} $ ). Friedel-Crafts alkylation is the name for this reaction.
A Friedel-Crafts reaction is a type of organic coupling reaction that uses an electrophilic aromatic substitution to attach substituents to aromatic rings. The Friedel-Crafts alkylation process is an electrophilic aromatic substitution reaction that adds an alkyl group to a benzene molecule.
Friedel-Crafts Alkylation is defined as the substitution of an alkyl group for an aromatic proton. With the help of a carbocation, an electrophilic attack on the aromatic ring is carried out. The Friedel-Crafts alkylation reaction uses alkyl halides as reactants to generate alkylbenzenes.
In this reaction, a Lewis acid catalyst such as $ AlC{l_3} $ or $ FeC{l_3} $ is used to facilitate the elimination of the halide and so generate a carbocation. Before proceeding with the alkylation step, the resultant carbocation undergoes a rearrangement. Toluene is formed as the final product.
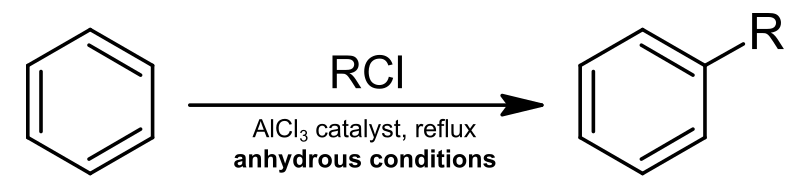
The reaction is as follows:
Here $ R $ represents $ C{H_3} $
Note :
Benzene is converted to alkyl benzene when it is treated with an alkyl halide in the presence of anhydrous aluminium chloride. The hydrogen atom of benzene is replaced by an alkyl group in this process. Alkyl groups are electrophiles, and aluminium plays a role in generating these electrophiles.
Complete Step By Step Answer:
Toluene is formed when benzene interacts with chloromethane ( $ C{H_3}Cl $ ) in the presence of anhydrous aluminium chloride ( $ AlC{l_3} $ ). Friedel-Crafts alkylation is the name for this reaction.
A Friedel-Crafts reaction is a type of organic coupling reaction that uses an electrophilic aromatic substitution to attach substituents to aromatic rings. The Friedel-Crafts alkylation process is an electrophilic aromatic substitution reaction that adds an alkyl group to a benzene molecule.
Friedel-Crafts Alkylation is defined as the substitution of an alkyl group for an aromatic proton. With the help of a carbocation, an electrophilic attack on the aromatic ring is carried out. The Friedel-Crafts alkylation reaction uses alkyl halides as reactants to generate alkylbenzenes.
In this reaction, a Lewis acid catalyst such as $ AlC{l_3} $ or $ FeC{l_3} $ is used to facilitate the elimination of the halide and so generate a carbocation. Before proceeding with the alkylation step, the resultant carbocation undergoes a rearrangement. Toluene is formed as the final product.
The reaction is as follows:
Here $ R $ represents $ C{H_3} $

Note :
Benzene is converted to alkyl benzene when it is treated with an alkyl halide in the presence of anhydrous aluminium chloride. The hydrogen atom of benzene is replaced by an alkyl group in this process. Alkyl groups are electrophiles, and aluminium plays a role in generating these electrophiles.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




