
What happens when ethyl chloride is treated with \[AgN{O_2}\] ? Write structure of the product?
Answer
496.8k+ views
Hint: Ethyl chloride is an ethane molecule having a chloride group attached to one of its two carbon atoms in place of a hydrogen atom. On the other hand \[AgN{O_2}\] is known as silver nitrate and is an important chemical compound in many reactions.
Complete answer:
\[AgN{O_2}\] is an important and commonly used chemical compound.
So, we will see its reaction with the ethyl chloride, so let’s write the balanced chemical equation of the reaction between the ethyl chloride and the silver nitrate, \[AgN{O_2}\] :
\[C{H_3} - C{H_2}Cl + AgN{O_2} \to C{H_3} - C{H_2}N{O_2} + AgCl\]
So, as we can see from the above reaction that when ethyl chloride reacts with the silver nitrate , it results in the production of ‘Nitro ethane ‘ and also in addition to it a silver colored compound – silver chloride is also produced as a by-product.
So, it is clear from the above discussion that ‘nitro ethane’ is produced in reaction of ethyl chloride with silver nitrate.
So, now also let’s have a look at the structural formula of the ‘nitro ethane’ molecule.
Therefore, the structure is:
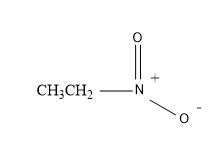
So from the above figure, the structure of the nitro ethane is crystal clear and is easy to understand.
Therefore, the correct answer is ‘silica’.
Note:
Also from the above discussion and from the structure of the ‘Nitro ethane’ we can say that the reaction between ethyl chloride and silver nitrate follows the \[{S_{N2}}\] mechanism of attack. Also note that the silver chloride is also produced as the silver precipitate in the above reaction.
Complete answer:
\[AgN{O_2}\] is an important and commonly used chemical compound.
So, we will see its reaction with the ethyl chloride, so let’s write the balanced chemical equation of the reaction between the ethyl chloride and the silver nitrate, \[AgN{O_2}\] :
\[C{H_3} - C{H_2}Cl + AgN{O_2} \to C{H_3} - C{H_2}N{O_2} + AgCl\]
So, as we can see from the above reaction that when ethyl chloride reacts with the silver nitrate , it results in the production of ‘Nitro ethane ‘ and also in addition to it a silver colored compound – silver chloride is also produced as a by-product.
So, it is clear from the above discussion that ‘nitro ethane’ is produced in reaction of ethyl chloride with silver nitrate.
So, now also let’s have a look at the structural formula of the ‘nitro ethane’ molecule.
Therefore, the structure is:

So from the above figure, the structure of the nitro ethane is crystal clear and is easy to understand.
Therefore, the correct answer is ‘silica’.
Note:
Also from the above discussion and from the structure of the ‘Nitro ethane’ we can say that the reaction between ethyl chloride and silver nitrate follows the \[{S_{N2}}\] mechanism of attack. Also note that the silver chloride is also produced as the silver precipitate in the above reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




