
Heating of carboxylic acid with soda lime results in:
A. dehydration
B. dehydrogenation
C. decarboxylation
D. addition of ${{{O}}_2}$
Answer
558.6k+ views
Hint: Carboxylic acid is an organic compound having $ - {{COOH}}$ group. The chemical formula of soda lime is ${{CaHNa}}{{{O}}_2}$. We know that the soda lime is obtained when calcium oxide is mixed with sodium hydroxide.
Complete step by step answer:
Soda lime is used in respiration. It eliminates carbon dioxide and prevents carbon dioxide poisoning. It is used as a drying agent. It absorbs carbon dioxide.
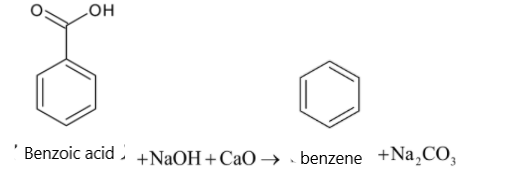
Soda lime is the mixture of ${{NaOH}}$ and ${{CaO}}$. But it is written as sodium hydroxide in equations. Sodium hydroxide removes ${{C}}{{{O}}_2}$ in a carboxylic acid molecule which produces alkane, i.e benzene. And the ${{C}}{{{O}}_2}$ reacts with sodium hydroxide to give sodium carbonate. The function of ${{CaO}}$ is that it makes ${{NaOH}}$ less reactive.
Decarboxylation reaction is a chemical reaction in which carbon dioxide is removed from any compound. While carboxylation is the addition of carbon dioxide to any compound. Decarboxylation reaction is possible only if there is a carboxyl group in the compound.
When a carboxylic acid is heated with soda lime, it undergoes decarboxylation. The carboxyl group in carboxylic acid is converted to sodium carbonate. It also forms a hydrocarbon. The chemical reaction is given below:
${{RCOOH}} + {{NaOH}}\xrightarrow[\Delta ]{{{{CaO}}}}{{RH}} + {{N}}{{{a}}_2}{{C}}{{{O}}_3}$
$\text{Carboxylic acid Hydrocarbon}$
So when carboxylic acid is heated with soda lime, the reaction is known as decarboxylation.
So, the correct answer is Option C.
Note: Sodium salts of carboxylic acids can also undergo decarboxylation reaction by heating them with soda lime. This also gives the same product as carboxylic acids. Let’s take an example. When sodium benzoate is heated with soda lime, it produces benzene, sodium carbonate and water molecules. The reaction is given below:
Complete step by step answer:
Soda lime is used in respiration. It eliminates carbon dioxide and prevents carbon dioxide poisoning. It is used as a drying agent. It absorbs carbon dioxide.
Soda lime is the mixture of ${{NaOH}}$ and ${{CaO}}$. But it is written as sodium hydroxide in equations. Sodium hydroxide removes ${{C}}{{{O}}_2}$ in a carboxylic acid molecule which produces alkane, i.e benzene. And the ${{C}}{{{O}}_2}$ reacts with sodium hydroxide to give sodium carbonate. The function of ${{CaO}}$ is that it makes ${{NaOH}}$ less reactive.
Decarboxylation reaction is a chemical reaction in which carbon dioxide is removed from any compound. While carboxylation is the addition of carbon dioxide to any compound. Decarboxylation reaction is possible only if there is a carboxyl group in the compound.
When a carboxylic acid is heated with soda lime, it undergoes decarboxylation. The carboxyl group in carboxylic acid is converted to sodium carbonate. It also forms a hydrocarbon. The chemical reaction is given below:
${{RCOOH}} + {{NaOH}}\xrightarrow[\Delta ]{{{{CaO}}}}{{RH}} + {{N}}{{{a}}_2}{{C}}{{{O}}_3}$
$\text{Carboxylic acid Hydrocarbon}$
So when carboxylic acid is heated with soda lime, the reaction is known as decarboxylation.
So, the correct answer is Option C.
Note: Sodium salts of carboxylic acids can also undergo decarboxylation reaction by heating them with soda lime. This also gives the same product as carboxylic acids. Let’s take an example. When sodium benzoate is heated with soda lime, it produces benzene, sodium carbonate and water molecules. The reaction is given below:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




