
Hexane and 3-methyl pentane are examples of:
A.Enantiomers
B.Stereoisomers
C.Diastereomers
D.None of the above
Answer
501.9k+ views
Hint: Hexane and 3-methyl pentane are Alkanes containing 6 carbon atoms each. The molecular formula of hexane is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}$ and 3-methyl pentane is $C{{H}_{3}}C{{H}_{2}}CH(C{{H}_{3}})C{{H}_{2}}C{{H}_{3}}$. Both have the same chemical formula that is ${{C}_{6}}{{H}_{14}}$. However, their structural formulas are different from each other.
Complete answer:
We know that Hexane and 3-methyl pentane have the same chemical formula but different structural formulas.
Let us understand the chain form of both the compounds:
a) Hexane is a long chain compound with all the 6 carbon atoms bonded to each other by single bonds.
\[C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_2} - C{H_3}\]
b) 3-methyl pentane: This is a branched-chain compound with one methyl group attached to the 3rd carbon of the pentane chain. The 3rd carbon atom in the chain becomes the tertiary carbon as it is attached to three other carbon atoms.
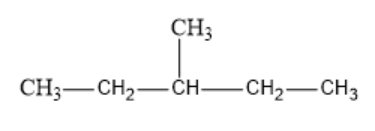
So Hexane and 3-methyl pentane show differences in the length of the carbon chain. Therefore, Hexane and 3-methyl pentane are Chain isomers.
Final answer: The correct Option is D- None of the above.
Additional information: Racemic mixture makes the compound optically active. The compounds which show optical activity form enantiomers. Stereoisomers are compounds that have the same molecular formula but have the difference in the spatial orientation of the groups attached to the parent chain. Enantiomers and Diastereomers are types of Stereoisomers.
Enantiomers are compounds that are non-superimposable mirror images of each other. However, Diastereomers are non-superimposable without being mirror images of each.
Note:
Chain isomerism falls under the category of Structural isomerism. It is also commonly known as skeletal isomerism considering the parent carbon chain as the skeleton of the structure. The chain isomers have different IUPAC names, although they have the same chemical formulas.
Complete answer:
We know that Hexane and 3-methyl pentane have the same chemical formula but different structural formulas.
Let us understand the chain form of both the compounds:
a) Hexane is a long chain compound with all the 6 carbon atoms bonded to each other by single bonds.
\[C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_2} - C{H_3}\]
b) 3-methyl pentane: This is a branched-chain compound with one methyl group attached to the 3rd carbon of the pentane chain. The 3rd carbon atom in the chain becomes the tertiary carbon as it is attached to three other carbon atoms.

So Hexane and 3-methyl pentane show differences in the length of the carbon chain. Therefore, Hexane and 3-methyl pentane are Chain isomers.
Final answer: The correct Option is D- None of the above.
Additional information: Racemic mixture makes the compound optically active. The compounds which show optical activity form enantiomers. Stereoisomers are compounds that have the same molecular formula but have the difference in the spatial orientation of the groups attached to the parent chain. Enantiomers and Diastereomers are types of Stereoisomers.
Enantiomers are compounds that are non-superimposable mirror images of each other. However, Diastereomers are non-superimposable without being mirror images of each.
Note:
Chain isomerism falls under the category of Structural isomerism. It is also commonly known as skeletal isomerism considering the parent carbon chain as the skeleton of the structure. The chain isomers have different IUPAC names, although they have the same chemical formulas.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




