
How do you draw a Hess’s law diagram?
Answer
535.5k+ views
Hint: The Hess’s law is major in physical chemistry which states that the total enthalpy change for a reaction is independent of the route by which the chemical change takes place. Formation of heat is defined as the enthalpy change which accompanies the formation of substance in a standard state from its elements also taken in standard state.
Complete step by step solution:
As we know that Hess law takes the independent route for the reaction. In thermodynamics, we need the initial and final states. So, we need to construct a Hess Cycle using the given information;
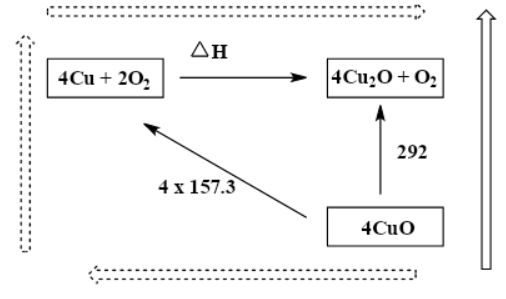
While we observe the diagram, in terms of energy, the dashed long route is equal to the black arrow route as the arrows start and finish in the same place. This diagram represents and follows the Hess law. So, we can write as; $(4\times 157.3)+\Delta H=292$
Therefore, $\Delta H=-337.2kJ$
We have found $\Delta H$ for below equation; \[4Cu+2{{O}_{2}}\to 2C{{u}_{2}}O+{{O}_{2}}\]
This means the same ; \[4Cu+{{O}^{2}}\to 2C{{u}_{2}}O\text{ }\]
This means the formation of two moles of copper(I) oxide. So, we need to find the change in enthalpy for the formation of copper(I) oxide. Therefore,
\[\Delta {{H}_{f}}\left[ C{{u}_{2}}O \right]\text{=}\dfrac{\Delta H}{2}=-\dfrac{337.2}{2}=-168.6\text{ }kJ/mol\text{ }\]
So, for the formation of one mole of copper(I) oxide we need \[-168.6\text{ }kJ/mol\text{ }\] enthalpy.
Note: We must know that Hess’s law is a version of the fine law of thermodynamics. So, it means the energy is always conserved. Often Hess’s law cycles are used to measure the change in enthalpy for a reaction that can’t be measured directly by experiments. Instead, the alternative method/ reactions are carried out that can be measured experimentally.
Complete step by step solution:
As we know that Hess law takes the independent route for the reaction. In thermodynamics, we need the initial and final states. So, we need to construct a Hess Cycle using the given information;

While we observe the diagram, in terms of energy, the dashed long route is equal to the black arrow route as the arrows start and finish in the same place. This diagram represents and follows the Hess law. So, we can write as; $(4\times 157.3)+\Delta H=292$
Therefore, $\Delta H=-337.2kJ$
We have found $\Delta H$ for below equation; \[4Cu+2{{O}_{2}}\to 2C{{u}_{2}}O+{{O}_{2}}\]
This means the same ; \[4Cu+{{O}^{2}}\to 2C{{u}_{2}}O\text{ }\]
This means the formation of two moles of copper(I) oxide. So, we need to find the change in enthalpy for the formation of copper(I) oxide. Therefore,
\[\Delta {{H}_{f}}\left[ C{{u}_{2}}O \right]\text{=}\dfrac{\Delta H}{2}=-\dfrac{337.2}{2}=-168.6\text{ }kJ/mol\text{ }\]
So, for the formation of one mole of copper(I) oxide we need \[-168.6\text{ }kJ/mol\text{ }\] enthalpy.
Note: We must know that Hess’s law is a version of the fine law of thermodynamics. So, it means the energy is always conserved. Often Hess’s law cycles are used to measure the change in enthalpy for a reaction that can’t be measured directly by experiments. Instead, the alternative method/ reactions are carried out that can be measured experimentally.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




