
How do you draw optical isomers?
Answer
558.6k+ views
Hint:somers are the molecules having the same chemical formulae but there is a difference in the arrangement of their atoms or groups in space.Two main types of isomers are stereoisomers and constitutional isomers.In the case of the stereoisomers, the bond connectivity is the same but different special arrangement groups or atoms of the compound.In the case of the constitutional isomers bond connectivity is different.Optical isomers are one of the types of stereoisomers that are optically active.
Complete step-by-step answer:The chiral center also called the asymmetric center is important in the case of the optical isomers. It is the center that is attached to four different groups.While drawing the optical isomers it is important to determine the chiral center.One of the methods used to draw the optical isomers is the solid-wedge method.In this method groups in the plane are represented by the solid lines, groups above the plane are represented by the wedge line and the dotted line is used to represent the groups below the plane.
For example:
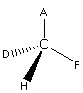
Here, the groups attached to the solid line are A and F which are in-plane. The group attached to the wedge line is H which is above the plane while the group attached to the dotted line is D is below the plane.Here, it is considered that the central atom C is attached to four different groups and it is a chiral molecule.The mirror image of the above molecule is as follows:
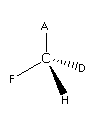
These two are optical isomers, one rorate the plane of the plane polarised light in one direction while others in the opposite direction.In this way, we can draw the optical isomers.
Note:Optical isomers are optically active that is which rotates the plane of the plane polarised light.The two types of optical isomers are enantiomers which are non-superimposable mirror images of each other and diastereomers are not mirror images of each other.In the case of the optical isomers, one rotates the plane of the plane polarised light clockwise while others rotate the plane of the plane polarised light anti-clockwise.
Complete step-by-step answer:The chiral center also called the asymmetric center is important in the case of the optical isomers. It is the center that is attached to four different groups.While drawing the optical isomers it is important to determine the chiral center.One of the methods used to draw the optical isomers is the solid-wedge method.In this method groups in the plane are represented by the solid lines, groups above the plane are represented by the wedge line and the dotted line is used to represent the groups below the plane.
For example:

Here, the groups attached to the solid line are A and F which are in-plane. The group attached to the wedge line is H which is above the plane while the group attached to the dotted line is D is below the plane.Here, it is considered that the central atom C is attached to four different groups and it is a chiral molecule.The mirror image of the above molecule is as follows:

These two are optical isomers, one rorate the plane of the plane polarised light in one direction while others in the opposite direction.In this way, we can draw the optical isomers.
Note:Optical isomers are optically active that is which rotates the plane of the plane polarised light.The two types of optical isomers are enantiomers which are non-superimposable mirror images of each other and diastereomers are not mirror images of each other.In the case of the optical isomers, one rotates the plane of the plane polarised light clockwise while others rotate the plane of the plane polarised light anti-clockwise.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




