
How do you name imines?
Answer
556.8k+ views
Hint: We know that imine is a chemical compound or a functional group possessing a double bond of carbon and nitrogen. An organic group (R) or a hydrogen (H) atom is attached to the nitrogen atom of the amine.
Complete step by step answer:
Let’s understand imines with the help of an example.
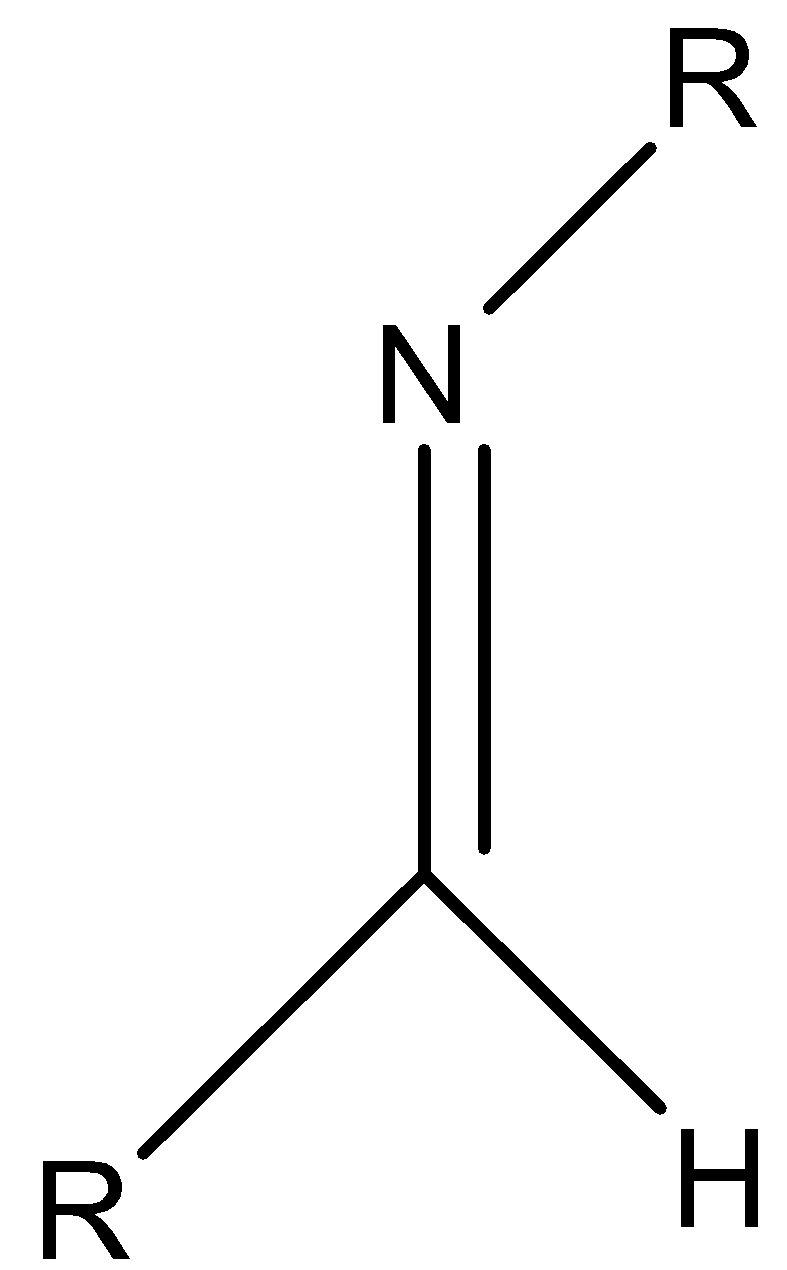
Here, there is a carbon nitrogen double bond present and N atom is attached to an organic group (R).
Let’s discuss the naming of imines. There are different ways of naming mines. In IUPAC naming of imines, we have to name the imines as the alkane substituted imine. That means, we have to add the suffix ‘imine’ to the name of the parent hydrocarbon chain. Let’s understand this rule by taking some examples.
The imine is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}} = {\text{NH}}$
Here, the parent hydrocarbon chain contains six carbon atoms. So, the name of the imine is Hexan-1-imine.
Another imine is ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_2} = {\text{NH}}$
Here, the parent carbon chain consists of three carbon atoms. So, the name of the imine is propan-2-imine.
Note: Let’s discuss imine derivatives in detail. When aldehydes and ketones react with ammonia formation of imine derivatives takes place. This imine derivative is also called Schiff base. The rate of formation of the imine derivatives is higher near a pH of 5 and its formation drops at lower and higher pH'S.
Another method of naming of imines is naming substitutively as ‘ylidene’ derivatives of parent hydride azane or by replacing the final ‘e’. For example, ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}} = {\text{NH}}$ imine is named as Hexylideneazane.
Complete step by step answer:
Let’s understand imines with the help of an example.

Here, there is a carbon nitrogen double bond present and N atom is attached to an organic group (R).
Let’s discuss the naming of imines. There are different ways of naming mines. In IUPAC naming of imines, we have to name the imines as the alkane substituted imine. That means, we have to add the suffix ‘imine’ to the name of the parent hydrocarbon chain. Let’s understand this rule by taking some examples.
The imine is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}} = {\text{NH}}$
Here, the parent hydrocarbon chain contains six carbon atoms. So, the name of the imine is Hexan-1-imine.
Another imine is ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_2} = {\text{NH}}$
Here, the parent carbon chain consists of three carbon atoms. So, the name of the imine is propan-2-imine.
Note: Let’s discuss imine derivatives in detail. When aldehydes and ketones react with ammonia formation of imine derivatives takes place. This imine derivative is also called Schiff base. The rate of formation of the imine derivatives is higher near a pH of 5 and its formation drops at lower and higher pH'S.
Another method of naming of imines is naming substitutively as ‘ylidene’ derivatives of parent hydride azane or by replacing the final ‘e’. For example, ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}} = {\text{NH}}$ imine is named as Hexylideneazane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




