
How will you convert the following?
Answer
565.5k+ views
Hint: Aromatic compounds are those compounds which contain one or more than one rings with delocalized $\pi $ electrons. Benzene is the most common aromatic compound having the chemical formula ${{C}_{6}}{{H}_{6}}$. Aromaticity is not only based on benzene rings but also defined by $(4n+2)\pi $ rule named as Huckel’s rule.
Complete Solution :
Chlorobenzene is an aromatic organic compound represented by the chemical formula ${{C}_{6}}{{H}_{5}}Cl$. It is generally a colorless, flammable liquid which is commonly used as a solvent and intermediate in the manufacture of many chemical reagents. On the other hand aniline is also an organic compound represented by the chemical formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$. It is also kept in the category of aromatic compounds in which phenyl group is attached with the amino group. Aniline is mainly used in the manufacturing of dyes and other industrial chemicals. It has a rotten fish-like smell.
Conversion of chlorobenzene to aniline can be done by step by step shown in the following reaction:
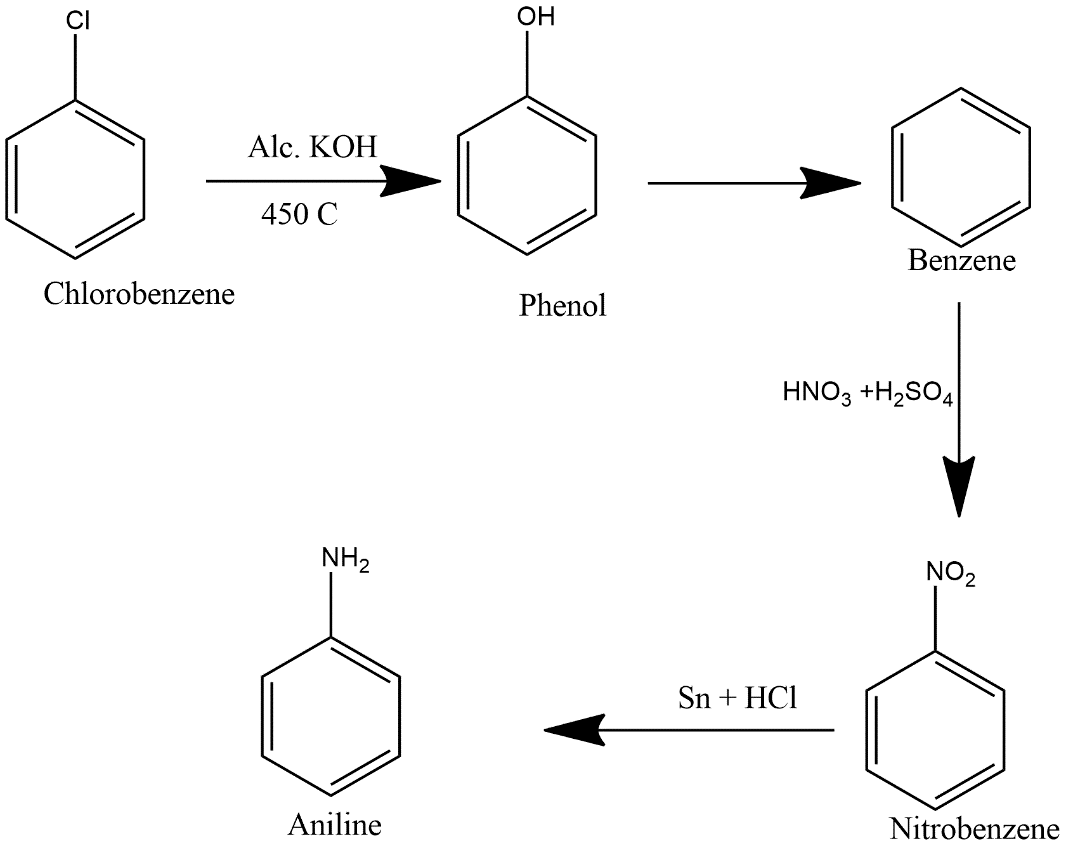
In the first step chlorobenzene reacts with an alcoholic form of $KOH$ and heated at $450{}^\text{o}C$ and converts into phenol, then phenol further reacts with zinc dust and converts into benzene ring. After this benzene is reacted with nitric acid and sulfuric acid represented by $HN{{O}_{3}}\ \text{and}\ {{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}$ respectively and benzene converted into nitrobenzene and then nitrobenzene reacts with $Sn\ \text{and}\ \text{HCl}$ and form aniline which is the required product.
Note: Aniline can be further diazotized which gives diazonium salts which can further undergo through the process of diazonium salts which further go through the process of nucleophilic substitution reactions. Aniline acts as a base like other amines.
Complete Solution :
Chlorobenzene is an aromatic organic compound represented by the chemical formula ${{C}_{6}}{{H}_{5}}Cl$. It is generally a colorless, flammable liquid which is commonly used as a solvent and intermediate in the manufacture of many chemical reagents. On the other hand aniline is also an organic compound represented by the chemical formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$. It is also kept in the category of aromatic compounds in which phenyl group is attached with the amino group. Aniline is mainly used in the manufacturing of dyes and other industrial chemicals. It has a rotten fish-like smell.
Conversion of chlorobenzene to aniline can be done by step by step shown in the following reaction:

In the first step chlorobenzene reacts with an alcoholic form of $KOH$ and heated at $450{}^\text{o}C$ and converts into phenol, then phenol further reacts with zinc dust and converts into benzene ring. After this benzene is reacted with nitric acid and sulfuric acid represented by $HN{{O}_{3}}\ \text{and}\ {{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}$ respectively and benzene converted into nitrobenzene and then nitrobenzene reacts with $Sn\ \text{and}\ \text{HCl}$ and form aniline which is the required product.
Note: Aniline can be further diazotized which gives diazonium salts which can further undergo through the process of diazonium salts which further go through the process of nucleophilic substitution reactions. Aniline acts as a base like other amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




