
How would you explain ionic radii?
Answer
528.9k+ views
Hint :We know that ionic radii are the radius of a monatomic ion in an ionic crystal structure. Ionic radius is the value assigned to the radius of an ion in a crystalline solid, assuming that the ions are spherical in nature with definite size. The ionic radii are the plural form of the ionic radius.
Complete Step By Step Answer:
We know that the removal of an electron from an atom results in the formation of a cation, whereas the gain of electron leads to the formation of an anion.
So, the ionic radii can be defined as the distance of the outermost shell of an anion or cation from its nucleus. For example, the bond length $ {{d}_{A-B}} $ between an ionic molecule is given as;
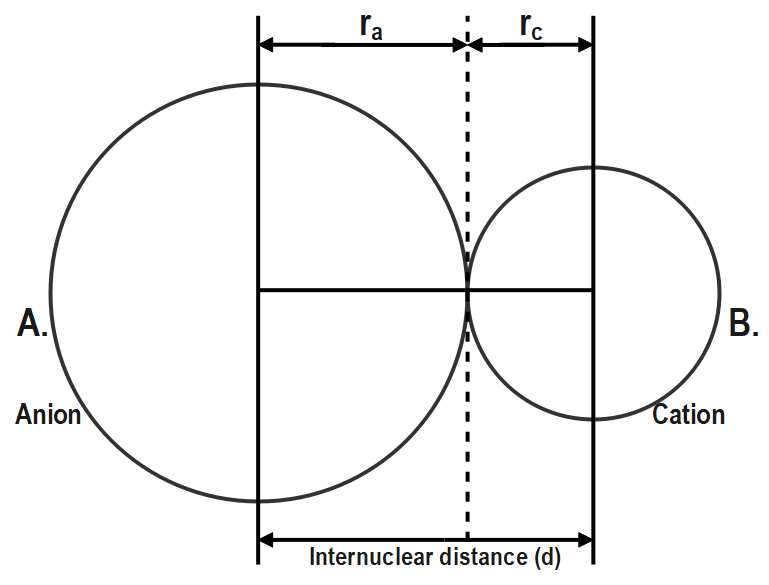
In the above figure $ {{r}_{a}} $ represent the anionic radius and $ {{r}_{c}} $ represent the cationic radius and the bond length AB is given as $ {{d}_{A-B}}={{r}_{c}}+{{r}_{a}} $ . Anionic radius and cationic radius are types of ionic radius.
In general, the ionic radii of elements exhibit the same trend as atomic radii.
Now, let’s talk about the Cationic and Anionic radii and concept of their size.
The radius of cation is always smaller than its parent atom because the cation is formed after the loss of electron has fewer electrons. Though the nuclear charge remains the same, the effective nuclear charge increases. So, the remaining electrons are pulled more strongly towards the nucleus, thus reducing the size of the cation.
The radius of anion is always larger than the parent atom because the anion formed by the gain of an electron has more electrons. The addition of one or more electrons results in the increased repulsion among the electrons and decreased intern clear charge, which in turn causes increased radii.
Note :
Ionic radii are typically given in units of either picometre (pm) or angstroms $ \left( {{A}^{\circ }} \right) $ . We should remember that $ 1A=100pm $ . Typical values range from $ 31 $ pm to over $ 200 $ pm. The concept of ionic radii can be extended to solvated ions in liquid solutions taking into consideration solvation shells.
Complete Step By Step Answer:
We know that the removal of an electron from an atom results in the formation of a cation, whereas the gain of electron leads to the formation of an anion.
So, the ionic radii can be defined as the distance of the outermost shell of an anion or cation from its nucleus. For example, the bond length $ {{d}_{A-B}} $ between an ionic molecule is given as;

In the above figure $ {{r}_{a}} $ represent the anionic radius and $ {{r}_{c}} $ represent the cationic radius and the bond length AB is given as $ {{d}_{A-B}}={{r}_{c}}+{{r}_{a}} $ . Anionic radius and cationic radius are types of ionic radius.
In general, the ionic radii of elements exhibit the same trend as atomic radii.
Now, let’s talk about the Cationic and Anionic radii and concept of their size.
The radius of cation is always smaller than its parent atom because the cation is formed after the loss of electron has fewer electrons. Though the nuclear charge remains the same, the effective nuclear charge increases. So, the remaining electrons are pulled more strongly towards the nucleus, thus reducing the size of the cation.
The radius of anion is always larger than the parent atom because the anion formed by the gain of an electron has more electrons. The addition of one or more electrons results in the increased repulsion among the electrons and decreased intern clear charge, which in turn causes increased radii.
Note :
Ionic radii are typically given in units of either picometre (pm) or angstroms $ \left( {{A}^{\circ }} \right) $ . We should remember that $ 1A=100pm $ . Typical values range from $ 31 $ pm to over $ 200 $ pm. The concept of ionic radii can be extended to solvated ions in liquid solutions taking into consideration solvation shells.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




