
What is hybridization? Explain $s{{p}^{3}}$ hybridization with an example.
Answer
567k+ views
Hint: Mixing of two different entities to get another entirely different entity is the general meaning of the term hybridization. The concept is also followed by atomic orbitals. Then, depending on the number and type of orbitals involved, there are different types of hybridization.
Complete Solution :
Hybridization is defined as mixing of two atomic orbitals of same or nearly same energies to get new orbitals of equivalent energy. These new orbitals are called hybrid orbitals. However, certain rules are followed while hybridization of orbitals occurs. These are as follows –
- Only central atom orbitals can undergo hybridization.
- To form a new hybrid orbital, participating atomic orbitals should be of the same energy.
- The number of hybrid orbitals formed is always equal to the sum of the number of participating atomic orbitals.
- The hybrid orbitals are scattered in space and tend to be farthest apart.
- Hybridised bonds are stronger than non-hybrid ones.
$s{{p}^{3}}$ type of hybridization – When one s- and the p- orbitals of the same atomic orbitals mix to give new hybrid orbitals of equivalent energy, $s{{p}^{3}}$ type of hybridization is observed.
Characteristic features of $s{{p}^{3}}$ type of hybridization are –
- The orbitals are directed towards four corners of a regular tetrahedron.
- All orbitals make an angle with each other.
- Each hybrid orbitals have 25% s character and 75% p character.
Simple example of $s{{p}^{3}}$ hybridization is ethane molecules.
- In ethane, $s,{{p}_{x}},{{p}_{y}}$ and ${{p}_{z}}$ orbitals of both the carbon atoms undergo $s{{p}^{3}}$ hybridization to give 4 hybrid orbitals with equal energy each. Among these orbitals, one hybrid orbitals of one carbon overlaps with 1s orbital of hydrogen and gives 3 sigma bonds. Last orbital overlaps with one $s{{p}^{3}}$ orbital of another carbon atom and gives a sigma bond between the two carbon atoms.
Similarly, $s{{p}^{3}}$ hybridization is observed in methane molecules. The $s,{{p}_{x}},{{p}_{y}}$ and ${{p}_{z}}$ orbitals of carbon atom undergo $s{{p}^{3}}$ hybridization to give 4 hybrid orbitals with equal energy. Among these orbitals, one hybrid orbitals of one carbon overlaps with 1s orbital of hydrogen and gives 4 sigma bonds. This is shown below,
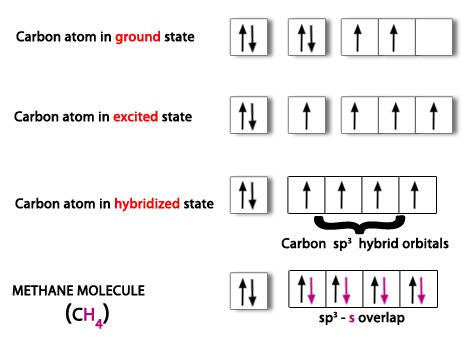
Note: Note that mixing of one s- orbital with 3 p- gives, $s{{p}^{3}}$ one s- and two p- orbitals gives $s{{p}^{2}}$ whereas. Mixing of one s- and one p- gives sp hybridization.
Complete Solution :
Hybridization is defined as mixing of two atomic orbitals of same or nearly same energies to get new orbitals of equivalent energy. These new orbitals are called hybrid orbitals. However, certain rules are followed while hybridization of orbitals occurs. These are as follows –
- Only central atom orbitals can undergo hybridization.
- To form a new hybrid orbital, participating atomic orbitals should be of the same energy.
- The number of hybrid orbitals formed is always equal to the sum of the number of participating atomic orbitals.
- The hybrid orbitals are scattered in space and tend to be farthest apart.
- Hybridised bonds are stronger than non-hybrid ones.
$s{{p}^{3}}$ type of hybridization – When one s- and the p- orbitals of the same atomic orbitals mix to give new hybrid orbitals of equivalent energy, $s{{p}^{3}}$ type of hybridization is observed.
Characteristic features of $s{{p}^{3}}$ type of hybridization are –
- The orbitals are directed towards four corners of a regular tetrahedron.
- All orbitals make an angle with each other.
- Each hybrid orbitals have 25% s character and 75% p character.
Simple example of $s{{p}^{3}}$ hybridization is ethane molecules.
- In ethane, $s,{{p}_{x}},{{p}_{y}}$ and ${{p}_{z}}$ orbitals of both the carbon atoms undergo $s{{p}^{3}}$ hybridization to give 4 hybrid orbitals with equal energy each. Among these orbitals, one hybrid orbitals of one carbon overlaps with 1s orbital of hydrogen and gives 3 sigma bonds. Last orbital overlaps with one $s{{p}^{3}}$ orbital of another carbon atom and gives a sigma bond between the two carbon atoms.
Similarly, $s{{p}^{3}}$ hybridization is observed in methane molecules. The $s,{{p}_{x}},{{p}_{y}}$ and ${{p}_{z}}$ orbitals of carbon atom undergo $s{{p}^{3}}$ hybridization to give 4 hybrid orbitals with equal energy. Among these orbitals, one hybrid orbitals of one carbon overlaps with 1s orbital of hydrogen and gives 4 sigma bonds. This is shown below,

Note: Note that mixing of one s- orbital with 3 p- gives, $s{{p}^{3}}$ one s- and two p- orbitals gives $s{{p}^{2}}$ whereas. Mixing of one s- and one p- gives sp hybridization.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




